A pilot study demonstrating the evidence for reflux disease in patients presenting with non-allergic rhinitis (NAR)—reflux disease in association with non-allergic rhinitis
Introduction
Non-allergic rhinitis (NAR) is a growing, adult onset disease (1) most common between patients aged 30–60 years (2). The disease affects up to 30% of individuals in the Western population (3). Thirty percent of all rhinitis cases are diagnosed to be NAR despite being poorly understood due to the convoluted, non-recognizable mechanisms which trigger the reaction (4). NAR is often described through a series of chronic nasal symptoms including obstruction, rhinorrhoea and postnasal drip (4-6). These heterogeneous symptoms occur in relation to non-allergic and non-infectious triggers such as environmental changes including the weather, exposure to caustic odours, cigarette smoke and barometric pressure differences (1).
The backflow of gastric contents including acid and pepsin into the esophagus is termed gastroesophageal reflux (GER) (6-8). Today, we recognize that reflux reaches beyond the esophagus causing extra-esophageal symptoms such as laryngitis, asthma and chronic cough, defined as extra-esophageal reflux (EER) or laryngopharyngeal reflux (LPR) (9). LPR is viewed as a subtype of gastroesophageal reflux disease (GERD) by gastroenterologists or a very different disease amongst ear nose and throat (ENT) and respiratory specialists. It has been found that many reflux induced extra-esophageal symptoms including hoarseness, throat clearing and postnasal drip (10) are very similar to those of patient’s suffering from NAR. Due to these similarities in symptoms, it is believed that LPR is an aetiology and increasing contribution towards diagnosis of NAR (4), however to date there is no data present about the role of reflux in this condition.
We aim to confirm the hypothesis and to evaluate the possible association between GERD and NAR with a non-invasive in-vitro diagnostic tool, Peptest. This study will be performed using clinical samples of patient’s saliva and nasal lavage.
Methods
Recruitment
Thirty-one patients (22 males and 9 females) were recruited during their ENT outpatient visits to The Affiliated Jinling Hospital of Nanjing University Medical School and The Affiliated BenQ Hospital of Nanjing Medical University upon their consent. All patients were presenting typical NAR clinical symptoms and underwent nasal endoscopy. An allergen skin prick test and CT scan of the sinuses were conducted to rule out allergic rhinitis and sinusitis respectively. Demographical characteristics were taken for 16 of the 31 patients (Table 1).
Table 1
| Parameter | N=31 |
|---|---|
| Male/female | 22/9 |
| Age range | 21–60 |
| Average age | 32 |
| Height range (cm) | 155–182 |
| Weight range (kg) | 50–85 |
| Eosinophil count range | 0–90 |
| Participants with a history of smoking | 3 |
| Participants with a history of drinking | 1 |
| Participants that prefer spicy cuisine | 8 |
| Failure to provide additional information | 13 |
A healthy control group (n=42, 11 males and 31 females) was recruited from Tongji Hospital at Tongji University School of Medicine, providing they achieved a score of 0 on completion of the Reflux Disease Questionnaire (RDQ). The age ranged between 22–66 years and the mean and median ages were 37 years and 34 years respectively (Table 2).
Table 2
| Parameter | N=42 |
|---|---|
| Male/female | 11/31 |
| Age range | 22–66 |
| Average age | 37 |
| Participants with a history of smoking | 6 |
Questionnaire
All participants were asked to complete the Reflux Disease Questionnaire (RDQ) (11,12).
Salivary pepsin
Collection
All participants (patients and control group) provided one postprandial saliva sample, with the patients suspected of NAR providing a further three samples. These included two additional saliva samples taken in the morning and after any patient self-reported symptoms and one nasal lavage sample collected on attendance to the clinic. Participants were informed not to take any medication during the study period and not to consume caffeinated or carbonated drinks 60 minutes prior to providing a sample.
Any morning samples were collected on waking before eating and cleaning teeth, provided postprandial; (pp) 60 minutes after finishing a meal and finally the third sample was provided 15 minutes after experiencing symptoms.
All the saliva and nasal samples were stored and refrigerated at 4 °C before being analyzed for the presence of pepsin using Peptest (RD Biomed Limited, UK).
Each sample was collected in a 30 mL collection tube containing 0.5 mL, 0.01 M citric acid, which acted as an antibacterial agent and stabilized the sample at an acidic pH to prevent pepsin auto-digestion (13).
Analysis
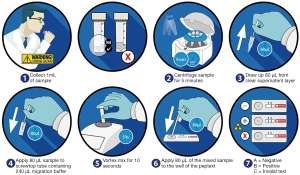
Collection tubes were centrifuged at 4,000 rpm for 5 minutes until a clear supernatant layer was visible. If not, the samples were centrifuged again, and 80 µL from the surface layer of the supernatant sample was drawn up into an automated pipette. The 80 µL sample was transferred to a screw-top microtube containing 240 µL of migration buffer solution. This sample was mixed on a vortex mixer for 10 seconds. A second pipette was used to transfer 80 µL of the sample to the circular well of a lateral flow device (LFD) (Figure 1) containing two unique human monoclonal antibodies; one to detect and the other to capture pepsin in the saliva or nasal lavage samples (Peptest, RD Biomed Limited, UK).
Fifteen minutes after introducing the clinical sample for pepsin analysis into the well of the Peptest, the lateral flow device was placed into the Peptest reader to determine the intensity of the pepsin test line. Pepsin concentrations >25 ng/mL were considered positive.
Eosinophil count
Collection
A clinician gently pushed a swab through the nose of each NAR presenting patient into the nasopharynx whilst rotating; ensuring an adequate amount of nasal lavage was collected.
Analysis
Swabs were rolled onto glass microscope slides transferring the specimen. The specimen slide was dried and dipped into CAMCO STAIN for 10 seconds before being transferred into distilled water for 20 seconds.
The specimen slide was placed under a microscope and observed through a high dry lens and morphology confirmed using a high oil lens before referring to Clinical Haematology Atlas (14).
Eosinophil counts less than 5% were considered normal.
Statistical analysis
Data was determined as mean and standard deviation (SD). Statistics were calculated using GraphPad Prism 7. For comparisons, an unpaired t test was carried out to determine P values (Figure 2) with a Pearson test of correlation and a Chi-square test performed where appropriate.
Results
Pepsin was detected in all 31 NAR patients in at least one sample provided. A higher level of pepsin was found in the postprandial sample (mean 142 ng/mL) compared to the morning sample (mean 67 ng/mL), P=0.0126. Pepsin levels greater than 25 ng/mL, were detected in 22 subjects (70.97%) in the postprandial test and 13 (41.94%) in the morning sample, suggesting that food has an effect on inducing reflux and increasing pepsin levels within the body. Of all the subjects, 1 patient (3.23%) had positive Peptest results for all four samples, 11 patients (35.48%) had positive results for 3 out of 4 samples, a further 11 patients (35.48%) were positive in two of the samples and 8 (25.81%) had tested positive in only one sample.
Pathological concentrations of pepsin were considered to be greater than 75 ng/mL. A total of 39/93 (41.94%) saliva samples and 4/31 nasal lavage samples (12.9%) were greater than this concentration in the patient samples. In the healthy control group there were 16 out of 42 positive postprandial saliva samples (38.1%). Fifteen of these positive saliva samples (35.7%) had a concentration greater than the 75 ng/mL cut off.
Not one NAR patient scored negatively for pepsin detection in all 4 of the samples. The average age for those who were determined positive in at least three of the samples was slightly lower than those who obtained positive in a maximum of two samples (29 versus 39 years) proving that age was not an essential cofactor in this study.
The mean RDQ score for NAR patients was 5.16 with only two subjects with a score equal to or greater than 12—the cut-off point for GERD diagnosis, Table 3.
Table 3
| Average response ranges | Total number of responses |
|---|---|
| 0–2 | 10 |
| 3–5 | 8 |
| 6–8 | 6 |
| 9–12 | 6 |
| 13+ | 1 |
| Mean response | 5.16 |
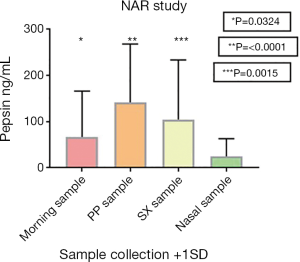
Fifteen of thirty-one (48.39%) nasal irrigation fluid samples analyzed for the presence of pepsin were positive. The mean pepsin concentration for the nasal lavage sample group was low at 25 ng/mL. The mean pepsin concentrations in the three saliva samples were all significantly different (P=0.0324, <0.0001 and 0.0015) to the mean pepsin concentration seen in the nasal irrigation fluid sample (Table 4). Figure 2 is a graphical representation of the pepsin concentration found in NAR patients present in this study.
Table 4
| Parameters | Number of participants with samples within the concentration range | |||
|---|---|---|---|---|
| Morning | Postprandial | After symptom | Nasal irrigation | |
| Concentration ranges (ng/mL) | ||||
| 0–50 | 17 | 10 | 15 | 26 |
| 51–100 | 5 | 5 | 4 | 4 |
| 101–150 | 4 | 2 | 4 | 0 |
| 151–200 | 0 | 4 | 3 | 1 |
| 201–250 | 2 | 3 | 1 | 0 |
| 251–300 | 1 | 3 | 1 | 0 |
| 301–350 | 0 | 2 | 1 | 0 |
| 351–400 | 1 | 1 | 0 | 0 |
| 401+ | 0 | 1 | 2 | 0 |
| Test failure | 1 | 0 | 0 | 0 |
| Mean concentration (ng/mL) | 67 | 142 | 105 | 25 |
| Standard deviation | 99 | 126 | 129 | 38 |
| P values* | 0.0324 | <0.0001 | 0.0015 | N/A |
*, in cases where the subject did not provide additional demographical data, they were recorded as a neutral value (0), with the amount of failure to complete subjects stated.
An eosinophil count was taken for all 31 NAR patients and their score recorded. The mean response for the number of eosinophils was high with an abnormal value of 23.48; however 18 of 31 subjects (58.06%) had a count within the normal, healthy range (Table 5).
Table 5
| Average response ranges | Total number of responses |
|---|---|
| 0 | 1 |
| 1–4 | 18 |
| 5–10 | 2 |
| 11–30 | 2 |
| 31+ | 8 |
| Mean response | 23.48 |
The comparison between the healthy control group and NAR patient postprandial saliva samples displayed visual differences. Greater quantities of positive samples were detected in postprandial patient samples (22/31) compared to the healthy control group (n=15). Similarly as predicted, the healthy control group had 27 negative samples whereas the patient population had 9.
The mean concentration in the postprandial samples of the NAR patient group compared to the healthy control group is shown in Table 6.
Table 6
| Groups | Mean response (ng/mL) |
|---|---|
| NAR patients (n=31) | 142 |
| Healthy control (n=42) | 45 |
A Chi-square test was performed and sensitivity, specificity, positive predictive values (PPV) and negative predictive values (NPV) were determined, Table 7.
Table 7
| Variables | % |
|---|---|
| Sensitivity | 87.1 |
| Specificity | 61.9 |
| PPV | 62.8 |
| NPV | 86.7 |
Discussion
As the link between NAR and reflux disease is yet to be confirmed, a key indicator whether there is a strong association is through the detection of pepsin. As pepsin is a digestive enzyme that is only produced in the stomach, it is detectable due to its large size (6,7). As a result of pepsin being generated in one location within the body (12), concentrations found within nasal and saliva samples is strong evidence that reflux is taking place, potentially resulting in GERD, LPR and respiratory diseases.
By using Peptest, a lateral flow device designed to detect the presence of pepsin, it displayed many of the test samples contained the enzyme pepsin (Table 4). This suggested that reflux and pepsin may be the contributing factor for a common clinical condition like NAR where the exact cause is still unknown. Therefore, the data gathered in the study shows the benefit of testing NAR patients for the presence of GERD and LPR.
As LPR is often the underlining cause for all rhinitis cases, the progression and development of NAR implies the patient has being suffering from LPR related diseases for an extensive period of time. This may potentially cause unnecessary distress due to the incorrect treatment being used (2,15).
Due to pepsin being a digestive enzyme, it is expected that food intake will cause an increased effect in its concentration, during the digestion process (16). This expectation is emphasized by the results from patient’s postprandial samples which are calculated to be significantly different to their morning samples (Figure 2), with a P value of 0.0126. Furthermore, 16 out of 42 healthy control samples had a positive result for pepsin, with 15 of these samples having a positive result >75 ng/mL. This further demonstrates food intake and diet can have an increased effect towards the levels of pepsin found within the body following a reflux event. Research has found the level of pepsin present within a sample is dependent on the time of collection and the symptoms experienced prior to the sample being taken (17), potentially affecting the results generated causing the significant difference in NAR patient samples to be present.
As eosinophils are normally found within the lamina propria, their presence in the epithelia of the GI tract implies a pathological condition (GERD) is present (18). Through analyzing the percentage of eosinophils in the NAR subjects, it will imply whether the patient is experiencing GERD due to the abnormal eosinophil count detected.
As there is not a visual correlation between those with an abnormal eosinophil count and those with a high pepsin concentration in the nasal lavage sample, a Pearson correlation was performed to further demonstrate the absence of correlation (r=−0.015).
Twenty one of 36 (58.33%) abnormal eosinophil samples were positive for pathological pepsin levels in comparison to 18 of 56 (32.14%) normal eosinophil levels. Despite this difference, they were not deemed to be significantly different (P=0.9450).
High pepsin sensitivity (87.1%) and specificity (61.9%) was determined using Peptest. This demonstrates the benefits and ease of the non-invasive, lateral flow device, compared to the Reflux Disease Questionnaire used in this study. The RDQ results displayed the classic GERD syndrome-based diagnostic method is not a sensitive method to diagnose LPR with the mean score achieved (5.16) being considerably lower than the limit to determine GERD (≥12), resulting in 29 NAR patients potentially being misdiagnosed for GERD and LPR.
As there was an absence of a clear visual difference between NAR patients who used alcohol and those who smoke in any of the samples provided, statistical data was not performed on these categories.
The data generated in the study demonstrated that there was a high level of pepsin in patient saliva samples compared to the healthy control population, providing a clear link and association of reflux disease in patients presenting with NAR. The effects of age, diet including spicy cuisine and eosinophil count were not contributing factors in this study.
The ease of using Peptest provided a rapid and efficient method with all subjects displaying positive for reflux disease due to the significant concentration of pepsin present, specifically in the postprandial saliva sample.
Physicians treating patients with NAR should consider reflux treatment for patients presenting with high pepsin concentrations.
Acknowledgments
We acknowledge the contribution of the individual participants who took part in the study.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoe.2019.03.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards (Ethical Approval ID: 2017NZKY-008-02). Informed consent was obtained from all individual participants included in the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Scarupa MD, Kaliner MA. Nonallergic rhinitis, with a focus on vasomotor rhinitis: clinical importance, differential diagnosis, and effective treatment recommendations. World Allergy Organ J 2009;2:20-5. [Crossref] [PubMed]
- Kaliner MA. Classification of Nonallergic Rhinitis Syndromes With a Focus on Vasomotor Rhinitis, Proposed to be known henceforth as Nonallergic Rhinopathy. World Allergy Organ J 2009;2:98-101. [Crossref] [PubMed]
- Hampel H, Abraham NS, El-Serag HB. Meta-Analysis: Obesity and the Risk for Gastroesophageal Reflux Disease and Its Complications. Ann Intern Med 2005;143:199-211. [Crossref] [PubMed]
- Wang Q, Ji J, Xie Y, et al. Lower airway inflammation and hyperresponsiveness in non-asthmatic patients with non-allergic rhinitis. J Thorac Dis 2015;7:1756-64. [PubMed]
- Turley R, Cohen SM, Becker A, et al. Role of Rhinitis in Laryngitis: Another Dimension of the Unified Airway. Ann Otol Rhinol Laryngol 2011;120:505-10. [Crossref] [PubMed]
- Gelardi M, Ventura MT, Fiorella R, et al. Allergic and non-allergic rhinitis in swimmers: clinical and cytological aspects. Br J Sports Med 2012;46:54-8. [Crossref] [PubMed]
- Bardhan KD, Strugala V, Dettmar PW. Reflux revisited: Advancing the Role of Pepsin. Int J Otolaryngol 2012;2012:646901 [Crossref] [PubMed]
- Ocak E, Kubat G, Yorulmaz I. Immunoserologic pepsin detection in the saliva as a non-invasive rapid diagnostic test for laryngopharyngeal reflux. Balkan Med J 2015;32:46-50. [Crossref] [PubMed]
- Ozmen S, Yücel OT, Sinici I, et al. Nasal Pepsin Assay and pH Monitoring in Chronic Rhinosinusitis. Laryngoscope 2008;118:890-4. [Crossref] [PubMed]
- Eren E, Arslangoǧu S, Aktaş A, et al. Factors confusing the diagnosis of laryngopharyngeal reflux: the role of allergic rhinitis and inter-rater variability of laryngeal findings. Eur Arch Otorhinolaryngol 2014;271:743-7. [Crossref] [PubMed]
- Shaw M, Dent J, Beebe T, et al. The Reflux Disease Questionnaire: a measure for assessment of treatment response in clinical trials. Health Qual Life Outcomes 2008;6:31. [Crossref] [PubMed]
- Harnik IG. In the Clinic: Gastroesophageal Reflux Disease. Ann Intern Med 2015;163:ITC1. [Crossref] [PubMed]
- Kim TH, Lee KJ, Yeo M, et al. Pepsin detection in the sputum/saliva for the diagnosis of gastroesophageal reflux disease in patients clinically suspected atypical gastroesophageal reflux disease symptoms. Digestion 2008;77:201-6. [Crossref] [PubMed]
- Clinical Haematology Atlas [computer program]. Version. Philadelphia: Saunders Elsevier, 2009.
- Johnston N, Dettmar PW, Strugala V, et al. Laryngopharyngeal reflux and GERD. Ann N Y Acad Sci 2013;1300:71-9. [Crossref] [PubMed]
- Luebke KE, Samuels TL, Johnston N. The Role of Pepsin in LPR: Will It Change Our Diagnostic and Therapeutic Approach to the Disease? Curr Otorhinolaryngol Rep 2016;4:55-62. [Crossref]
- Hayat JO, Gabieta-Somnez S, Yazaki E, et al. Pepsin in saliva for the diagnosis of gastro-oesophageal reflux disease. Gut 2015;64:373-80. [Crossref] [PubMed]
- Ayazi S, Hagen JA, Chandrasoma P, et al. Esophageal Intraepithelial Eosinophils in Dyspahgic Patients with Gastroesophageal Reflux Disease. Dig Dis Sci 2010;55:967-72. [Crossref] [PubMed]
Cite this article as: Wang Q, Lenham RK, Wang X, Li Y, Jiang M, Chen W, Xu L, Yang C, Woodcock AD, Dettmar PW. A pilot study demonstrating the evidence for reflux disease in patients presenting with non-allergic rhinitis (NAR)—reflux disease in association with non-allergic rhinitis. Ann Esophagus 2019;2:6.