
Physiologic and molecular effects of alcohol in the esophagus: a narrative review
Introduction
Background
Alcohol continues to be widely used and misused, accounting for a significant morbidity and mortality around the world, including a significant number of non-communicable diseases and injuries (1). Despite this, alcohol use continues to grow in several regions of the world, particularly lower-middle-income countries, with forecast trends showing abstinence decreasing to 40%, the proportion of current drinkers increasing to 50%, and the percentage of adults who are heavy episodic drinkers continuing to increase to 23% globally by 2030 (1). Similar trends are present regionally and in the Americas. In high-income countries, the total volume of alcohol consumed has remained stable, with predictions that these countries’ contribution to global alcohol use will be halved by 2030 (from 42% in 1990 to 26% in 2017 to 19% in 2030). In the USA, however, ongoing decreases in life expectancy and increases in mortality rates have been attributed to alcohol (2).
Rationale and knowledge gap
Although the esophagus is one of the first organs to be exposed to alcohol, remarkably little is known about the direct effects of alcohol on the human esophagus. The esophagus is exposed to a wide range of concentrations of ethanol in the setting of alcohol consumption. The concentration of ethanol in beer, for example, is 5%. In wine, it is 10% ethanol and high-proof alcoholic beverages such as whiskey, brandy, sherry, and gin can have an ethanol concentration as high as 40% (3). The esophagus is directly exposed to these concentrations of alcohol, and in individuals with esophageal motility disorders, the time of exposure to high concentrations of ethanol may be prolonged. Radiographic studies have demonstrated that radiolabeled boluses can remain in the esophagus for up to 2 hours after swallowing (4). Prolonged ethanol exposure may also occur, e.g., in the esophagus of individuals who sip alcoholic beverages with meals. For perspective, the legal limit for alcohol levels in the blood or the blood alcohol level (BAL) in the USA is 0.08% or 17.3 mM and can be achieved after 4–5 drink equivalents (5). The esophagus, however, is initially directly exposed to much higher concentrations of alcohol via oral ingestion, as the ingested alcohol passes through to the stomach.
The esophageal mucosa is locally exposed, however, not only to alcohol but to the damaging effects of its toxic metabolite acetaldehyde (6,7). Ethanol absorbed in the stomach and duodenum into the bloodstream is metabolized in the liver mostly by alcohol dehydrogenase (ADH) with some additional contribution by cytochrome P-4502E1 (CYP2E1) and catalase to an intermediate and toxic metabolite, acetaldehyde. CYP2E1 is also induced by ethanol intake (6,7). In the liver, acetaldehyde is subsequently metabolized mainly by mitochondrial aldehyde dehydrogenase 2 (ALDH2), which has a high affinity for acetaldehyde, to the non-toxic acetate. Acetate is then oxidized to produce carbon dioxide, fatty acids, and water in peripheral tissues (6,7). Local and potentially toxic accumulation of acetaldehyde may also be driven by a decrease in ALDH2 activity in the setting of chronic alcohol intake (8). Additional differences in enzyme activity may also drive acetaldehyde accumulation. In the oral epithelium, e.g., human class III alcohol dehydrogenase (ADH3) and human class IV alcohol dehydrogenase (ADH4), affinity for alcohol is low, meaning a lot of substrate is required to saturate the enzyme; meanwhile, CYP2E1, which is otherwise not normally expressed, is induced with chronic ethanol use and the amount of ALDH is negligible (6). These alterations can lead to toxic levels of acetaldehyde in the oro-esophagus. Ethanol can also be recirculated via the bloodstream to the salivary glands and tissues such as the esophagus (6). Alcohol can also be directly oxidized to acetaldehyde by oral cavity microorganisms, which then have limited ability to further metabolize the acetaldehyde to acetate, leading to its accumulation in the saliva (7,9).
After ingestion, ethanol directly contacts the esophageal mucosa; in this setting, once inside esophageal cells, there is also evidence to suggest that ethanol can also be metabolized to acetaldehyde (6,8). The direct topical effects of ethanol or acetaldehyde on squamous epithelial cells of the esophagus have previously been considered to be limited, with the thought being that the effect is limited to superficial cells of the epithelium (6). However, this rationale fails to account for instances in which ethanol exposure may be prolonged, for example, with chronic sipping of an alcoholic beverages; it also fails to account for numerous instances in which alcohol consumption occurs in the multitude of disorders with a disrupted esophageal barrier is disrupted [e.g., as in gastroesophageal reflux disease (GERD), obesity, or eosinophilic esophagitis] (10-13).
Objective
This article comprehensively reviews the known effects/associations of alcohol use on the human esophagus, beyond its known association with esophageal squamous cell cancer (ESCC). Alcohol is certainly considered a risk factor for esophageal squamous cell carcinoma, but has also been reported as a risk factor for reflux symptoms and GERD. We review associations between alcohol and esophageal cancer and explore the potential mechanisms, including what is known about alcohol-related cellular signaling and the microbiome in the esophagus prior to the development of malignancy. We also review what has been described regarding effects of alcohol on esophageal motility and any association with increase in reflux symptoms and mechanisms. Finally, we explore areas that are ripe for future research. This article is presented in accordance with the Narrative Review reporting checklist (available at https://aoe.amegroups.com/article/view/10.21037/aoe-24-46/rc).
Methods
We performed a literature search using PubMed, Google Scholar, and Medline databases between April 8, 2024, and December 1, 2024. To identify all relevant literature, the following search terms were used: “Alcohol OR ethanol” AND “esophagus OR GERD OR gastroesophageal reflux OR reflux OR cancer OR EoE OR eosinophilic esophagitis OR signaling OR epithelium OR stroma OR fibrosis OR microbiome OR signaling”. Studies published in English with full available text were included. Publication date filters or article type filters were not utilized (Table 1). Studies focused on the esophagus and utilizing esophageal tissues were prioritized. References listed in the publications that were identified by search terms were also reviewed and included if relevant.
Table 1
| Items | Specification |
|---|---|
| Date of search | April 8, 2024–December 1, 2024 |
| Databases and other sources searched | PubMed, Google Scholar, Medline |
| Search terms used | “Alcohol OR ethanol” AND “esophagus OR GERD OR gastroesophageal reflux OR reflux OR cancer OR EoE OR eosinophilic esophagitis OR signaling OR epithelium OR stroma OR fibrosis OR microbiome” |
| Timeframe | From inception to December 1, 2024 |
| Inclusion criteria | Studies published in English were included. No limitation in the type of article |
| Selection process | A single academic clinician with an interest in esophageal disorders and the effect of alcohol on the esophagus, performed study selection |
Alcohol and the human esophagus
Alcohol and risk for esophageal malignancy
There is a dose-dependent, direct association between alcohol intake and an increased risk for a subtype of esophageal cancer, ESCC (14,15). Alcohol consumption has not been shown to be associated with an increased risk of another subtype of esophageal cancer, esophageal adenocarcinoma (EAC) or its precursor Barrett’s esophagus (BE) (16-19). The direct association between ESCC and alcohol has been demonstrated in a wide range of studies, including case-control, prospective cohort studies (20,21) as well as in a number of meta-analyses (22-27), and systematic reviews of published meta-analyses (28,29). While it is clear that the risk of ESCC increases with higher doses of alcohol, the threshold level below which alcohol consumption is safe in terms of risk of ESCC remains vague. This lack of clarity is driven in part by heterogeneity of methods used to quantify alcohol consumption across various studies as well role of additional established risks for ESCC such as genetic variants in enzymes responsible for ethanol metabolism and the inconsistent interaction with smoking (15). For example, even definitions of low, moderate, or excessive intake (30) have varied widely between studies. Moderate drinking, e.g., generally considered to be no more than 1 drink a day for women and 2 drinks a day for men, with a standard alcoholic drink considered to consist of ~14 gm of pure alcohol (30).
In an early meta-analysis that included 28 studies on esophageal cancer (not subtyped into ESCC or EAC) (22), a significantly increased risk esophageal cancer was found with increasing risk associated with higher intake, and independent of the effect of tobacco, starting at the lowest dose of alcohol that was considered, 25 gm a day (~2 drinks a day) with an adjusted relative risk (RR) [95% confidence interval (CI)] of 1.52 (1.46–1.57), 2.23 (2.09–2.38), and 4.31 (3.84–4.85) for 25, 50 and 100 g/day of alcohol, respectively (22).
A subsequent meta-analysis that included 27 studies focused on the risk of ESCC (9 cohort and 18 case-control) associated with light drinking (up to 1 drink/day or 1–12.5 gm ethanol/day) (23), demonstrated that even low alcohol intake significantly increases the risk of ESCC. The overall RR (95% CI) was 1.30 (1.09–1.56), while for men it was 1.46 (1.19–1.80) and for women 1.28 (0.84–1.96). The increased risk was evident when studies were stratified by sex or study type (cohort or case-control studies). When stratified according to geographical area, however, the increased risk of ESCC was only significant in studies of Asian populations with an RR 1.49 (1.12–1.98) (23). An increased risk of ESCC with light drinking was not observed in Europe or North America. Limitations of this meta-analysis included heterogeneity of the studies reporting on ESCC and lack of information on tobacco consumption.
A subsequent meta-analysis expanded on these prior meta-analyses and included 54 publications focused on the ESCC subtype, and also on the dose response relationships between alcohol consumption (where light drinking was considered ≤12.5 g/day, moderate drinking was ≤25 g/day, and heavy drinking ≥50 g/day). The overall RR (95% CI) of ESCC was 1.26 (1.06–1.50) for light drinking, 2.23 (1.87–2.65) for moderate drinking, and 4.95 (3.86–6.34) for heavy drinking (27).
The association between esophageal cancer and modifiable risk factors including alcohol consumption was also addressed in a systematic review of published meta-analyses that included 11 studies focused on the ESCC sub-type, and which included the above-described meta-analyses (28). Overall and sex-specific associations between alcohol drinking and the occurrence of ESCC for these 11 studies were reported. There were no significant differences between study type (case-control or cohort studies) in geographical areas. For individual studies that considered alcohol drinking habits (ever versus never), the overall RR (95% CI) for ever-drinkers versus never-drinkers was 1.36 (1.15–1.61) (26,28), while in a meta-analysis that considered sex-specific associations, the RR (95% CI) for ESCC in ever-drinkers versus never-drinkers was 3.70 (2.75–4.98) among men and 2.10 (1.32–3.35) among women (24,28). Dose-response effects identified in several meta-analyses were also reported (28). For studies that considered drinks per day (versus zero drinks/day), the higher the number of drinks per day, the higher the risk of ESCC. For example, in one study, the RR (95% CI) for 1–3 drinks/day versus 0 drinks/day was 2.56 (1.10–5.96), for 3–5 drinks/day it was 4.56 (2.32–8.96), for 5–7 drinks/day the RR was 7.17 (2.98–17.25) and for 7+ drinks/day the RR was 9.62 (4.26–21.72) (20,28). Similar trends were reported in another study that considered drinks/day (25,28) with a significant RR (95% CI) starting at 3–5 drinks per day of 2.15 (1.29–3.58), for 5–10 drinks/day an RR of 2.74 (1.47–5.10), and for 10+ drinks/day an RR of 4.12 (2.01–8.44). Similar dose-response patterns were observed across meta-analyses that quantified alcohol consumption as g pure ethanol/week (28,31), g pure ethanol/day (23,24,27,28,32), years of drinking (24,28), or drink-years (25,28).
Finally, a recent meta-analysis that included 44 case-control and cohort studies on ESCC reported the RR of ESCC for the highest level of alcohol intake versus the lowest level of alcohol intake was 5.11 (95% CI, 3.60–7.25), with significant associations observed across different alcoholic beverages, gender, and study type (29). The relationship between alcohol intake and ESCC risk was linear (Pnon-linearity=0.216) with an RR (95% CI) of 1.03 (0.94–1.13) for alcohol intake of 2 g/day, RR of 1.26 (0.89–1.77) for alcohol intake of 10.7 g/day, an RR of 1.87 (1.32–2.63) for alcohol intake of 22 g/day ESCC, an RR of 3.08 (2.2–4.30) for 40 g/day, up to 4.3 (3.00–6.16) for 65 g/day, with an increase in ESCC risk of 33% for every 12.5 g increment of daily alcohol intake (29). Risk of ESCC with different drinking patterns, including binge drinking type pattern, remains incompletely described (14).
The risk of EAC and alcohol intake was also addressed by 13 studies in this meta-analysis (29); the summary RR of EAC and highest versus lowest alcohol intake was 0.96 (95% CI, 0.79–1.16), with similar pattern across different alcoholic beverages, gender, and study type (29). Overall, alcohol consumption does not appear to be associated with an increased risk of EAC, while it is clearly associated with an increased risk of ESCC in a dose-dependent manner. Based on the available studies, which are limited by heterogeneity and differences in definitions/descriptions of alcohol use, although the threshold level below which alcohol consumption is safe remains unknown, it is clear that higher alcohol consumption increases the risk of ESCC.
Mechanisms of alcohol associated esophageal carcinogenesis
Although ethanol by itself was initially considered neither genotoxic or mutagenic (33), re-review of a number of animal studies utilizing ethanol alone and in studies in which co-administration of ethanol increased chemically induced carcinogenesis, prompted the International Agency for Research on Cancer (IARC) to recognize alcohol as a Group 1 carcinogen (9,34). Mechanisms of ethanol-associated esophageal carcinogenesis are likely multifactorial, and the molecular mechanisms involved have yet to be completely elucidated. However, a number of biological mechanisms may explain the relationship between alcohol consumption and esophageal cancer (35).
Acetaldehyde is a toxic metabolite of ethanol oxidation and is also considered a Class 1 carcinogen by the World Health Organization (33,34). Acetaldehyde is a highly reactive compound that forms deoxyribonucleic acid (DNA) adducts that induce DNA-DNA crosslinking, gene mutations, and double-strand DNA breaks, inhibition of DNA repair, with effects on epigenetics or DNA methylation (33,36). Acetaldehyde can be produced by micro-organisms in the oral cavity (7). Alcoholic beverages can also contain acetaldehyde, providing another source of direct exposure to acetaldehyde (7).
A number of studies have shown a strong association between ESCC and genetic polymorphisms in the primary enzymes involved in ethanol metabolism, ALDH and ADH (36). A meta-analysis of 15 studies showed that the risk of esophageal cancer is increased in those with a variant allele of the aldehyde dehydrogenase enzyme ALDH2 that results in reduced enzymatic activity and ability to oxidize acetaldehyde, and therefore higher blood acetaldehyde concentrations (37). A meta-analysis of 34 case-control studies showed that polymorphisms of alcohol dehydrogenase 1B (ADH1B) and ALDH2 were associated with the risk of esophageal cancer and that the risk was increased by alcohol consumption (38).
The role of ALDH2 and its expression in relation to alcohol consumption has been investigated in the human esophagus. Cytoplasmic and mitochondrial members of the ALDH family and CYP enzymes were evaluated in esophagectomy specimens of ESCC (8). There was strong staining for cytoplasmic ALDH1 and CYP1A1 in basal and parabasal squamous epithelial cells as well as in the lamina propria compartment of human esophagectomy specimens. ALDH2 was also expressed in human esophagus, although ALDH2 levels varied among individuals (8). This variability in ALDH2 was examined in a subsequent study of a larger number of esophagectomy specimens and correlated with alcohol drinking habits (39). Positive staining for ALDH2 was observed again in the cytoplasm of epithelial cells in the basal zone of squamous epithelium. Specimens with the highest expression of ALDH2 were all from alcohol drinkers, most of whom were characterized as heavy drinkers. Conversely, in specimens with the low or no expression of ALDH2, the incidence of heavy drinkers was much lower or not recognized. Thus, ALDH2 levels in the normal appearing esophageal epithelium of ESCC esophagectomy specimens appeared to be related to drinking status and suggested alcohol induced esophageal expression of ALDH2 (39).
ALDH2 expression in response to alcohol consumption has been further investigated in murine models. ALDH2 levels increased in the esophagus of mice treated with 10% ethanol in the drinking water for 8 weeks compared to the esophagus of mice without ethanol drinking (40). The increase in ALDH2 was observed in the basal and parabasal layers of the epithelium. Levels of the DNA adduct N2-ethylidene-dG, which reflects acetaldehyde-derived DNA damage, were also increased in the esophagus of ethanol versus water drinking mice. The increase in DNA damage was even more profound in the esophagus of mice in which the enzymatic activity of ALDH2 had been genetically knocked out compared to the levels of DNA damage in the esophagus of ethanol drinking mice in which ALDH2 activity was intact (40). In this study, direct treatment of a human esophageal epithelial cell line with doses of acetaldehyde (≤1 mM) that did not lead to significant decreases in cell viability for 72 h, induced DNA adduct formation and also increased ALDH2 messenger ribonucleic acid (mRNA) and protein levels production in a time and dose-dependent manner. Treatment with 1 mM acetaldehyde for 72 h also increased ALDH2 mRNA in esophageal epithelial cell lines and primary cells. Treatment with a range of acetaldehyde doses (0.2–1 mM) for 72 hours increased ALDH2 protein most profoundly at 1 mM; and treatment with acetaldehyde 0.2 mM for 0–72 hours showed a progressive increase in ALDH2 protein levels. Finally, ALDH2 depletion in human esophageal epithelial keratinocytes resulted in more DNA damage in response to treatment with acetaldehyde 0.2 mM for 72 hours than when ALDH2 was present. Conversely, overexpression of ALDH2 diminished acetaldehyde-induced DNA damage incurred with treatment with 0.2 mM acetaldehyde for 72 h in a human esophageal epithelial cell line. Interestingly, proliferative, and migratory activities of cells with acetaldehyde induced DNA damage in this study were not altered. Enzymatic activity of ALDH2 could also not be determined (40). Overall, the results provided further evidence for a role for ALDH2 in the esophagus, and suggested that in esophageal epithelial cells, ALDH2 could serve as a cytoprotective local defense against acetaldehyde-derived DNA damage (40).
Induction of the CYP2E1 system and generation of reactive oxygen species (ROS), particularly in the metabolism of high alcohol concentrations or in alcohol dependent patients, have also been implicated (33,36). Studies reporting on risks associated with CYP2E1 polymorphisms and ESCC risk and alcohol consumption have been variable. In a study with the Brazilian population, there was no risk associated with CYP2E1 and ESCC with alcohol consumption (41). In an Asian study, there was an increased risk of esophageal cancer in heavy drinkers carrying CYP2E1c1/c1 or CYP2E1c1/c2 genotypes versus non-drinkers (42). In all, a number of factors, related to alcohol consumption, including the role of acetaldehyde outlined above, along with roles for folate deficiency, coincident tobacco smoke, and polymorphisms of genes involved in ethanol metabolism, play a role in the development of ESCC (43).
Alcohol and the esophageal microbiome
16S rRNA sequencing has confirmed culture-based methods that show that the most common genera of bacteria of the native esophageal microbiome are Streptococcus, Prevotella, and Veillonella (44-46). In a cross-sectional study of 1,044 US adults, drinking alcohol and, in particular, heavy drinking were associated with shifts in diversity of oral microbiota and overall bacterial profiles that differed between drinkers and non-drinkers, including decreased abundance of Lactobacillales (47). A number of mechanisms have been proposed to explain the effects of alcohol on the oral microbiome, including the direct effect of ethanol on bacteria and bacterial metabolism of ethanol (47).
While the esophageal microbiome may be broadly similar to the oral microbiome, it may also have unique characteristics, particularly when considering disease states. Thus, while oral bacterial profiles have been considered as surrogate markers of esophageal microbiome, these profiles may not be representative of esophageal bacterial profiles, which may be more directly involved in esophageal disease. Notably, there is some evidence at least for EAC, e.g., that while tissue-associated microbiome (biopsy-derived) has an association with the disease state of the patient, non-biopsy derived microbiome, e.g., saliva or fecal microbiome, may not (48).
The effect of alcohol consumption has been evaluated in the microbiota in the saliva and in the esophagus in a population characterized by smoking and drinking status. Esophageal brush specimens from upper, middle, and lower segments of the esophagus were subjected to 16S rRNA gene profiling via next-generation sequencing (49). The most abundant phylum in the non-smoking/non-drinking population was Proteobacteria, while in the smoking/drinking group, Firmicutes was the most abundant phylum. In addition, differences in microbial diversity between the saliva and esophageal samples in the non-smoking/non-drinking group were lost in the smoking/drinking population (49). There were also differences in microbial abundance in the upper, mid, and distal esophageal levels of the smoking/drinking group versus the non-smoking/non-drinking group. Gemella levels in the upper esophagus were higher in the smoking/drinking versus non-smoking/non-drinking group, while in the middle esophagus, Bacteroides levels were higher in the non-smoking/non-drinking group. Levels of Rothia in the lower esophagus were higher in the smoking/drinking group than in the non-smoking/non-drinking group (49). Interestingly, differences in the abundances of bacteria usually associated with esophageal squamous cell carcinoma (e.g., Haemophilus, Neisseria, and Porphyromonas) were not observed between smoking/drinking and non-smoking/non-drinking populations (49). This observation prompted authors of the study to speculate whether there were factors beyond the microbiota were responsible for mediating the carcinogenic effects of alcohol (49), as it remains unclear whether the observed differences in the microbiota are an epiphenomenon versus involved in disease pathogenesis.
An interplay between a pro-inflammatory oral microbiome characterized by an increase in ADH activity with alcohol use that produces toxic acetaldehyde levels has been proposed in the development of oral squamous cell carcinomas (50). By extension, for example, a similar process could be in play in the development of ESCC. Interestingly, lower salivary microbial diversity has been shown to be associated with ESCC in a large case-control study in an area with high incidence of ESCC (51). Shifts in the esophageal microbiome, with a lower microbial richness, e.g., have been associated with esophageal squamous dysplasia, a precursor lesion for ESCC, in 333 samples from a Chinese cancer screening cohort (52). A number of case-control studies have also investigated changes in oral and/or esophageal microbiota in esophageal squamous cell carcinoma, with some showing a decrease in Streptococcus species and an increase in Porphyromonas gingivalis and Fusobacterium nucleatum (46).
Given the impact of alcohol on the oral and esophageal microbiomes, and the association between shifts in oral and esophageal microbiomes and risk for ESCC (46), the association between alcohol consumption and the esophageal microbiota has also been investigated in ESCC. In a hospital-based retrospective study of 120 patients in China with primary ESCC, alcohol consumption was associated with alterations in diversity and composition of the tissue-derived esophageal microbiota, suggesting that alterations in esophageal microbiota in alcohol drinkers may be involved in ESCC development (53). Mechanisms by which alcohol influences the esophageal microbiota, as well as mechanisms by which microbiome alterations may lead to ESCC, remain unknown and are under active investigation. For example, alcohol may lead to shifts in microbiota such that acetaldehyde levels increase as a consequence of differences in ethanol metabolism. Finally, the impact of alcohol drinking on the functional content of the esophageal microbiome merits investigation. Research in this sphere has been somewhat hampered by a lack of standardization of procedures for obtaining oral and/or esophageal samples.
Alcohol and signaling pathways in esophageal cells
Due to its dual hydrophilic and lipophilic properties, ethanol diffuses into squamous epithelial cells (54), leading to changes in the cell membrane and altering the function of intrinsic membrane proteins that can enhance the penetration of carcinogens across the epithelium (6). Studies across a wide range of tissues, cell types, and differing treatment concentrations and durations have demonstrated disruption of a number of signaling pathways in response to ethanol. The potential impact of ethanol on toll-like receptor 4 (TLR4), Notch, Sonic hedgehog (Shh), Wnt, NF-κB, transforming growth factor β (TGFβ), as well as other signaling pathways including mitogen-activated protein kinase, PI2K/Akt signaling and G-protein coupled receptors in squamous epithelial cells has been nicely reviewed (6).
The impact of ethanol on TLR4 signaling, for example, has been proposed to occur via alteration of the normal TLR4 complex in the plasma membrane lipid raft that typically occurs with LPS stimulation (55). In vitro treatment of human monocytes with 25 mM ethanol for 16 hr (a concentration of alcohol that occurs in humans after 3–4 standard drinks) inhibited TNF-α production after LPS (1 µg/mL) stimulation (56). These pathways have not been investigated in the esophagus. Interestingly, in a study of ESCC tumor samples, adjacent normal tissue, and normal controls, several TLRs were evaluated by immunohistochemistry and with quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) (57). TLR3, TLR4, TLR7, and TLR9 mRNA and protein were overexpressed in ESCC tumor samples compared to normal tissue, while TLR3 and TLR7 were mainly expressed in esophageal tumor cells. TLR4 was also found to be highly expressed in monocytes and TLR9 was found to be expressed in fibroblast-like cells (57).
A number of studies have shown that alcohol consumption can impact DNA methylation (58), which in turn can lead to changes in oncogene and tumor suppressor gene expression (9,58,59). Alcohol-induced alterations in gene expression due to deregulation of DNA methylation of the promoter region of chromosomes, e.g., have previously been described in a genome-wide DNA methylome analysis of human embryonic stem cells in culture treated with 20 or 50 mM ethanol for 24 or 48 hours (60). Epigenome-wide association studies have identified the promoter of the cystine/glutamate transporter SLC7A11 as the most alcohol-related methylation site (58,61). Interestingly, SLC7A11 or cystine transporter solute carrier family 7 member 11, is a cystine transporter that imports extracellular cystine that is built up in the setting of oxidative stress (62). Once imported into the cytosol, cystine is reduced to cysteine and then used to synthesize glutathione (GSH) for antioxidant defense. SCL7A11 can protect cells from oxidative-stress induced cell death and has increased expression in many cancers (62). Abnormal gene methylation, therefore, may in turn affect activity of genes in a number of relevant signaling pathways, e.g., Notch, PI3K/Akt and Wnt signaling pathways (6). Methylation changes have been reported in ESCC samples (63).
PAX9 is a member of the Pax family of transcription factors, essential to the normal development of multiple tissues, and expressed in squamous epithelial cells of the human esophagus. Downregulation of this transcription factor occurs in epithelial dysplasia and ESCC of the human esophagus (64). In a study that included human ESCC samples, a decrease in PAX9 expression in ESCC versus adjacent normal tissue was confirmed (65). Utilizing clinical data including alcohol exposure, the decrease in PAX9 expression was even more profound in ESCC tissue of alcohol drinkers versus non-drinkers. Interestingly, downregulation of PAX9-positive cells was also observed in the adjacent normal esophageal tissue of alcohol drinkers versus adjacent normal tissue from non-drinkers (65). Time and dose-dependent ethanol exposure also showed PAX9 down-regulation in a human ESCC cell line (65). PAX9 protein was decreased in cell lines starting with 50 mM ethanol after 72 hours of treatment. In cells treated with 100 mM (0.6%) ethanol, a decrease in PAX9 was observed after 48 hours of treatment. In mice exposed to 20% ethanol for 4 weeks or 15% ethanol for 40 weeks ethanol in drinking water, there was an increase in thickness of the squamous epithelium and an increase in cell proliferation and a decrease in squamous differentiation and a decrease in PAX9 expression (65). These changes were observed only in the mouse forestomach, which unlike humans, is also squamous epithelium, and were not observed in mouse esophagus. There were no differences in methylation levels as analyzed by pyrosequencing between control and ethanol-exposed esophageal cancer cell lines, suggesting alternative mechanisms are mediating PAX9 downregulation in this system (65). To our knowledge, methylation patterns in alcohol exposed, non-cancerous esophageal tissue, as in e.g., those individuals who have a history of chronic alcohol consumption (CAC) and or alcohol use disorder (AUD), have not yet been investigated.
In a follow-up study, the molecular mechanism by which ethanol suppressed PAX9 in the esophagus was investigated (66). Several up- and downregulated signaling pathways were identified in human esophageal cancer cell lines treated with 100 mM ethanol for 24 h using a commercially available reporter array and confirmed with subsequent immunoblot; and included downregulation of the NOTCH effectors c-MYC, and p-STAT3 (66). In addition, gene microarray analysis of treated cells showed inhibition of NOTCH signaling, and suppression of genes related to squamous differentiation. Ethanol via drinking water of 20% ethanol for 4 weeks or 15% ethanol for 40 weeks also inhibited NOTCH signaling in mouse squamous epithelium (66). In total, these findings showed that ethanol exposure inhibits PAX9 expression in human esophageal cancer cell lines in vitro and in vivo in mouse models via inhibition of NOTCH signaling (66). NOTCH signaling regulates development of esophageal squamous epithelium and squamous differentiation in the esophagus (67). Treatment of an immortalized human esophageal epithelial cell line with a variety of inflammatory cytokines, e.g., resulted in downregulation of/suppression of an activated form of NOTHC1, ICN1 (68). Genes in the NOTCH pathway are frequently mutated in human ESCC (67), although they may not be enough to drive carcinogenesis alone. The effect of ethanol on Notch signaling, however, varies in different organs and tissues and has not been expressly investigated in normal human esophagus exposed to alcohol.
Shh signaling is another important developmental signaling pathway. The effect of ethanol on this pathway differs in various organ systems; for example, ethanol inhibits Shh signaling in fetal alcohol syndrome, while in the liver, ethanol activates Shh signaling and promotes carcinogenesis (67). Interestingly, in zebrafish, alcohol exposure (0.5%) decreases Shh signaling with decreased expression of Shh target genes Ptc, Gli1, Nkx2.2 and also interferes with post-translational modification of Shh in the plasma membrane that is critical to its function (69). The effect of ethanol on Shh signaling was further studied in a hepatic stellate cell line (HSC8B) (70). Treatment with various concentrations of alcohol (0%, 0.2%, 0.4%, 0.6%, and 0.8%) for 2 h, interrupted the formation of a protein complex between Shh and caveolin-1 in lipid rafts, leading to cytoplasmic accumulation of Shh and prevention of its secretion (70). Ethanol also alters membrane-associated cholesterol, which is important for Shh signaling (71). In the esophagus, Shh is activated in esophageal cancer including in EAC, its precursor lesion BE (72) and in ESCC (73). Shh activation in ESCC suggests a role for ethanol in this pathway (67), although the effect of ethanol on Shh signaling in the normal human esophagus remains unexplored.
Similarly, the effect of ethanol on Wnt signaling is tissue-specific. In the esophagus, Wnt signaling activation is associated with advanced ESCC. Recent multi-omics analysis has demonstrated 6 major carcinogenesis tracks in early ESCC, with the smoking/drinking track 6 characterized by immune response, T cell receptor and interleukin (IL) signaling and antigen processing and presentation (73). In addition, in the context of ESCC, single-base substitution (SBS)16 is associated with alcohol drinking (74) and with OLFM4 mutation, which is relevant to DNA replication (73). In an immortalized laryngeal cell line exposed to various ethanol concentrations (0–1,000 mM) for 4 h, 24 h, or 48 h, ethanol affected cell survival in a dose- and time-dependent manner. Bulk RNA sequencing of cells treated with a wide range of ethanol concentrations showed that treatment with 250 mM (~1.5%) ethanol resulted in differential gene expression patterns with a broad effect on the transcriptome, including upregulation of Wnt signaling ligands and receptors such as Frizzled class receptors (75). Additional investigation of the effect of ethanol on Wnt signaling in non-malignant esophageal tissue/cells is pending.
The TGFβ signaling is another critical development pathway that is also implicated across a wide range of malignancies, including esophageal cancer (76). TGFβ is also a central mediator of fibroblast activation (77) including in eosinophilic esophagitis, wherein it leads to extracellular matrix (ECM) production (fibronectin, collagen), expression of α-smooth muscle actin and increased contractility (78). Adaptor proteins of the TGFβ signaling pathway, e.g., Smad 4 and β2-spectrin, may play a role in regulating DNA damage and alcohol-induced DNA damage, respectively, in models of head and neck cancer, fetal alcohol syndrome, and hepatocellular carcinoma (79). Treatment of rat cardiac fibroblasts with ethanol (0–400 mg/dL) for 24 h showed that ethanol doses of 100 (0.1% or 16 mM), 200 (0.2% or 34 mM) or 400 mg/dL (0.4% or 68 mM) induced α-SMA expression, contractility of fibroblasts seeded in collagen matrices, and migration that was blocked by TGFβ inhibition (80). Ethanol treatment of cardiac fibroblasts at these doses had no effect on apoptosis or proliferation (80). In addition, treatment with ethanol induced expression of collagen type 1 mRNA and protein, TGF-β mRNA, and also likely secretion of TGF-β, given that effects were inhibited by blocking TFGβ signaling via a soluble recombinant TGFβ receptor (80). These pathways have yet to be examined in the esophagus in the setting of alcohol exposure. The effect of direct alcohol treatment on esophageal stromal cell contractility or migration has not yet been determined. Studies in human esophageal cell lines exposed to ethanol for short durations of time in a cyclic manner, in an effort to mimic human consumption of alcoholic beverages, also suggest there may be activation of pro-inflammatory pathways, and that conditioned media from treated cells increased migratory capacity of surrounding cells (81). This study, however, was limited to cell lines with cyclic treatment that may have confounded the interpretation of the findings (81).
The effects of ethanol on the MAPK and P13/Akt signaling pathways are dependent on the cell type studied and whether ethanol administration was acute or chronic, and/or in the context of stimulation with additional factors. Both inhibition and activation of these pathways have been described in response to ethanol (6). The effects of alcohol on these signaling pathways remain unexplored in the human esophagus.
Studies evaluating the direct effects of alcohol and acetaldehyde on proliferation and apoptosis of esophageal cells have shown somewhat conflicting results, in part due to different sensitivities of various cell types as well as different models used. In addition, differences in treatment dose and duration have led to discrepant results. In a head and neck cancer cell line, incubation with ethanol 10−3 M or 0.001 M or 1 mM for 96 hours increased cellular proliferation as well as expression of IL-4, epidermal growth factor (EGF), and platelet-derived growth factor (PDGF) receptors (82). In an immortalized human laryngeal cell line, however, only exposure to the highest ethanol concentration 1,000 mM (~6%) for 4 hours resulted in minor decline in cell survival (75) whereas prolonged exposure to lower concentrations of ethanol decreased cell survival to below 85% survival (e.g., with 400 mM for 24 hours and 250 mM for 48 hours) (75). Additional cell lines were also examined including immortalized human esophageal epithelial cell lines, while esophageal epithelial cells were found to be generally more sensitive to ethanol than laryngeal cells, the observed effects of ethanol appeared to be in part cell-line dependent (75). A human esophageal epithelial cell line treated for 8 hours with 2% ethanol remained viable but with RNA sequencing showing broad transcriptomic effects, most notably effects on mitochondrial function (83). Three-dimensional (3D) human esophageal organoids derived from an immortalized human esophageal epithelial cell line and established in the presence of ethanol 100 mM (0.6%) from days 3 through day 10 were smaller in size than untreated organoids (83). Established esophageal organoids that were grown without ethanol for the first 9 days and then treated with or without ethanol (0.2%, 0.6%, 2%) for 24 h demonstrated a decrease in viability at 2%; while established organoids grown for various duration in the presence of 2% ethanol, only showed a decreased in viability and a decrease in size at in response to 24 h of treatment (83). Validation studies demonstrated evidence of mitochondrial dysfunction and oxidative stress, with cytoprotection against ethanol-induced stress suggested to occur via autophagy, a mechanism that clears damaged/dysfunctional mitochondria (83). In total, these studies show that the effect of alcohol is both dose and duration-dependent.
Transient receptor potential channel vanilloid subfamily 1 (TRPV1) is a nonselective cation channel (84) predominantly expressed in sensory neurons has also been described in the esophagus, including in epithelial and stromal cells (85). In addition to being a putative acid receptor, this polymodal receptor is activated by heat and some lipids. Treatment of sensory neurons from the rat esophagus with ethanol 0.1–3%, which is equivalent to 17–513 mM, led to a dose-response related increase in release of the neuropeptides substance P (SP) and CGRP. Neuropeptide release was inhibited by pre-treatment with capsaicin and the TRPV1 antagonist capsazepine and required the presence of extracellular Ca2+ ions (86). Overall, the study demonstrated that ethanol may have an effect on calcium transport and release of neuropeptides from rat sensory neurons via TRPV1 activation (86,87). The effect of alcohol on TRPV1 activity has not yet been evaluated in the other esophageal cells of the human esophagus.
There is also evidence to suggest that alcohol may perturb the host defense system with modulatory effects on the immune system in a dose and time-dependent manner (36). Alcohol may affect innate and adaptive immunity via effects on lipid rafts, receptor complexes and associated signaling (55). It’s plausible that alcohol consumption is related to tumor growth, e.g., via its perturbation of the immune system, including impaired anti-tumor regulation or recruitment of cells and cytokine generation that increase oxidative stress and reduce cell death (36), although these mechanisms have not yet been explicitly studied in ESCC. Alcohol’s effects on the immunological response have been evaluated in multiple tissues, including the brain, lung, liver (88). Studies of the effects on the esophagus are lacking, perhaps because of the perceived rapidity through which alcohol may pass through the esophagus. This rationale however, does not account for the chronic alcohol use, which incorporates sipping of alcoholic beverages throughout the day or with food or in those with esophageal motility disorders that impair transit. Regardless, the consequences of these impaired immune reactions in the development of esophageal diseases have not been investigated.
Overall, the molecular and biologic effects of alcohol are complex and are dependent on context, tissue type, concentration, and duration. In terms of signaling pathways related to mechanisms of carcinogenesis, in addition to potentially disturbing signaling pathways in squamous epithelial cells and the microenvironment, disturbances of metabolism in squamous epithelial cells and disturbances in systemic metabolism of nutrients such as retinol, zinc and iron have also been proposed as mechanisms of alcohol related carcinogenesis (6). The effects of ethanol on the human esophagus prior to the development of malignancy remain incompletely understood and additional work is required to tease out the direct effect of alcohol on normal human esophageal cells of the esophageal mucosa.
Alcohol and the esophageal epithelial barrier
The effect of alcohol exposure on alterations in esophageal mucosal tissue resistance has also been investigated in animal models. The effect of ethanol 1–40%, alone and in combination with other noxious agents, has been evaluated on the structure and function of rabbit esophageal epithelium in vitro and in vivo (89-91). Rabbit esophageal mucosa has structural and functional similarities to the human esophagus, although unlike human esophageal mucosa, rabbit esophagus does not have submucosal glands. Tissue morphology, dose and time dependent changes in potential difference (PD, which reflects transepithelial voltage and mucosal resistance and ion transport) and short-circuit current (which reflects epithelial transport, and which can be active transcellular Na+ transport or paracellular) were evaluated using Ussing chambers and used to determine electrical tissue resistance (an indicator of barrier integrity or function) in rabbit esophageal tissue continuously exposed to ethanol for 1 hour (89). Experiments done in this study showed the direct effects of ethanol on esophageal epithelial transport and barrier function and notably demonstrated changes even at the lowest concentrations. At concentrations of 1% (171 mM) and 5% (856 mM), continuous ethanol exposure, there were changes observed as early as 10 minutes after exposure (89). At 1% ethanol, there was a progressive increase in and eventual stabilization of PD and of short circuit current within 10 min, although resistance remained stable (89). At 5% ethanol, there was an increase in PD for the first 10 minutes followed by progressive decline back to baseline, a progressive increase in short-circuit current that occurred within 10 minutes, and a decrease in tissue resistance that started within 10 minutes and continued to decline over the remaining hour, without morphological changes after 1 hour (89). At concentrations of 10% (1,712 mM) ethanol, PD started and continued to decrease after 20 min of exposure, there was an initial increase in short-circuit current at 10 min followed by progressive decline, and a more profound decrease in resistance that started within 10 min, accompanied by more marked tissue edema (89). Interestingly, these changes were reversible within an hour of exposure to 5% ethanol for 5–30 min but not after exposure lasting 60 min; in addition, the changes were not reversible with 10% ethanol, with failure of the tissue to recover to its baseline resistance even after a 10 min exposure (89). At a 1% concentration of ethanol, the increase in short circuit current was abolished with pretreatment with ouabain, demonstrating that at low ethanol concentrations, the increase in short circuit current was dependent on active (transcellular) Na+ transport; while at 10% ethanol, the reduction in resistance was associated with an increase in mannitol flux suggesting the decrease in resistance was due to increase in permeability via the paracellular pathway (89). In addition, there was a dose-dependent decline in electrical resistance with continuous exposure to concentration of ethanol >1%. This decline occurred with minor effects on morphology for 5% and 10% ethanol but was associated with an increase in transepithelial mannitol flux (89). These findings suggested the change was due to an increase in paracellular permeability and likely an alteration in intercellular junctions. In other words, continuous exposure to ethanol leads to dysfunction of the esophageal epithelial barrier function. With 40% ethanol, changes in short circuit current occurred in the setting of tissue damage and visible edema (89). For perspective, beer typically has 5% alcohol by volume, table wine has 12% alcohol, liqueur has 24%, and scotch has 40% alcohol (3).
Additional in vivo experiments were performed in rabbits with intermittent administration of ethanol bolus to mimic human alcohol consumption (89). In vivo, PD measurements were similar to patterns observed in vitro with continuous ethanol exposure; for e.g., with 10% ethanol, the PD increases for the first 5–10 min then declines and falls to baseline by 30 min; this pattern was similar to that observed with 5% ethanol continuous exposure (89). Similarly, e.g., with 20% ethanol in vivo, there is an initial increase in PD followed by progressive decline (similar to what was seen with 10% continuous ethanol exposure in vitro); and similar to PD observed in vitro for 40% ethanol exposure for 1 hour, in vivo PD decreased rapidly and progressively to near abolishment within 10 min (89). Electrical parameters of this tissue were then evaluated in Ussing chambers, with a dose-dependent lowering in tissue resistance that became significant with 20% ethanol intermittent in vivo exposure (89). Morphologic changes with in vivo intermittent ethanol exposure included diffuse edema in response to 20% and 40% ethanol, without damage at 10%. Essentially, in vivo bolus ethanol exposure to slightly higher concentrations of ethanol mimicked continuous in vitro ethanol exposure, while 40% ethanol resulted in similar toxicity in vitro and in vivo studies (89). In summary, experiments in this study showed that in vitro exposure to ethanol at low concentrations 5% resulted in alterations ion transport and/or barrier function and that at higher concentrations (≥10%) there were morphological changes (89).
A later study confirmed that all concentrations of ethanol 1–10% increased the short circuit current, possibly by increasing ion transport and reducing tissue resistance (91). Notably, with the exception of tissue exposed to 1% ethanol, tissue exposed to ethanol concentrations of 2.5–10% for 60 minutes did not recover during a 45 washout phase and observed changes in current and resistance were irreversible (91). Marked reductions in PD (reflecting transepithelial voltage) in the human esophagus have also been reported after wine or whiskey consumption, with a decrease in esophageal PD after 250 mL of 11% red wine and 60 mL of 43% whisky (92). It is also important to note, as has been mentioned in prior investigations, that while pure ethanol, is usually a “drink” that is utilized in biomedical investigations, alcoholic beverages actually have additives that may also be contributing to observed effects (92).
Rabbit esophageal epithelium exposed to 10% ethanol, acid [hydrochloric acid (HCl), pH 2], or combinations of ethanol + acid, tissue injury has also been examined by measuring PD and electrical resistance, and evaluating histology (90). While sections of rabbit esophageal epithelial tissue exposed to acid alone for 1 h did not demonstrate significant changes, exposure to 10% ethanol for 1 hour lowered the PD and electrical resistance of the tissue and produced cellular edema in the upper layers (90). Esophageal tissue that was exposed to both ethanol and acid led to an even greater decrease in PD and morphologic damage including edema and necrosis (90). Interestingly, differences in esophageal tissue resistance were also noted with transient exposure to ethanol. For example, esophageal tissue that was treated with ethanol for 10 minutes, demonstrated a decline in tissue resistance over a 1-hour period, once the ethanol was removed. In addition, tissue that was pre-treated with ethanol for brief periods of only 10 min and subsequently exposed to acid, for up to 1 hour following ethanol removal also showed lower resistance and greater morphologic damage than ethanol treatment for 10 min alone or acid exposure up to 1 hour alone (90). Similar changes in PD or short circuit current were appreciated in pre-ethanol treated tissue even with delays of up to 1 hour before acidification pH 2 and even with less noxious concentrations of HCl at a pH 3 or 4 (90). These studies suggest that the effects of alcohol exposure and vulnerability to additional damage via e.g., endogenous irritants such as acid may remain even after alcohol clearance from the esophageal lumen. Alcohol may e.g., increase the risk of reflux esophagitis by impairing the epithelial tissue’s resistance to acid and thereby predisposing the tissue to acid-induced injury.
The additive effect of ethanol and an extract of cigarette smoke has also been investigated in rabbit esophagus epithelium using in vitro and in vivo models (91). Ethanol decreased tissue resistance and impaired the epithelial barrier while cigarette smoke altered ion transport; when applied or consumed together, the results were more pronounced. When ethanol and cigarette smoke were administered sequentially, ethanol predisposed the tissue to injury by cigarette smoke (91).
Effect of alcohol on esophageal motility
The effect of ethanol on esophageal motility has been studied in normal volunteers and those with AUDs. In one of the first studies to evaluate the effect of alcohol on esophageal motor function, 12 healthy individuals were administered oral (86 proof bourbon whiskey, 43% ethanol by volume) or intravenous injection (IV) (9.5% ethanol solution in 5% dextrose water) ethanol and esophageal manometric changes were evaluated (93). Acute intoxication with these doses of ethanol in otherwise normal, healthy individuals resulted in a decrease in lower esophageal sphincter pressure (LESP), a decrease in amplitude of distal esophageal contractions and a reduction in distal esophagus primary peristalsis. These changes were reversible, with normal esophageal motor function restored at 8 and 24 hours after ethanol administration (93).
Manometric changes have also been evaluated in chronic alcoholics (94). Esophageal manometry and esophageal radionuclide emptying were conducted on 18 alcoholics within 3 days of their last drink as well as after one month of sobriety (94). Lower esophageal sphincter (LES) resting pressure and esophageal contraction amplitudes were higher in alcoholics compared to controls; abnormal motility patterns were also observed in 78% or 14/18 alcoholic patients, including e.g., hypercontractile patterns such as nutcracker esophagus in 50% or 9/18 and associated with delayed esophageal emptying in 7/18 (94). Relaxation of the LES was normal in response to swallowing (primary peristalsis). Abnormal findings normalized with 1 month of abstinence (i.e., there was normalization of LESP, esophageal contraction amplitudes, and motility abnormalities) (94). Upper endoscopy and pinch biopsies of the distal esophagus were also performed in alcoholic subjects. The esophagus appeared normal in all patients and esophageal mucosal histology was normal in all except for 1 patient who had thickened basal layer and neutrophil infiltration (manometry and emptying study were normal in this patient) (94). These findings suggested that esophagitis that is observed in alcoholics is not related to motility abnormalities and that manometric abnormalities or motor disturbances observed in alcoholics may not necessarily be associated with functional abnormalities (e.g., delay in emptying) (94).
Esophageal motility was also evaluated in a study that included 13 normal healthy controls, 6 chronic alcoholics within 6 hours of their last drink, and 13 chronic alcoholics following 24–48 h of abstinence while signs of withdrawal were present (95). In normal healthy controls, intravenous administration of ethanol (0.8 g/kg) decreased LESP transiently, inhibited LES relaxation, and decreased esophageal contraction amplitudes (95). In withdrawing alcoholics, the effects of ethanol administration on the LES were less pronounced; in addition, esophageal contraction amplitudes were elevated and infusion of ethanol returned contraction amplitudes towards normal in withdrawing alcoholics (95). Esophageal motor abnormalities were frequently seen in the alcoholic subjects including nutcracker esophagus and hypertensive LES (95). Overall, this study demonstrated that primary or swallow-induced esophageal contractions are abnormal in alcoholics; it also showed the differing effects of acute alcohol administration, CAC, and alcohol withdrawal on esophageal motility (95).
The effect of ethanol on secondary esophageal contractions, a mechanism that clears refluxed material (e.g., acid), has also been studied (96). Esophageal motility in response to wet swallows (primary peristalsis) and to intra-esophageal injection of acid or saline (secondary peristalsis) was evaluated in 19 male alcoholics. Overall, secondary esophageal contractions were abnormal in both actively drinking and withdrawing alcoholics (96).
Twenty-three chronic alcoholic patients were evaluated by endoscopy, manometry, and 24 h pH monitoring 7–10 days and 6 months after withdrawal of ethanol (97). High amplitude contractions have been reported in the middle third of the esophagus in chronic alcoholics (mean intake 266 g/day over 24 years) (97). Sixty-one percent had reflux symptoms. On endoscopy, most had a normal appearing esophagus (17/23), with 5 out of those with a normal appearing esophagus with histologic inflammation, while 5 out of 23 had endoscopic evidence of esophagitis (97). One individual had asymptomatic ESCC that presented as 3 cm polypoid lesion in the upper esophagus. A percent reflux time >2.9% was found in 52%, and LESP was hypertensive in 57%. In addition, there were differences in peristaltic metrics compared to age and sex matched control groups, with high amplitude contractions in the middle third of the esophagus (>150 mmHg) a distinguishing feature (97). This observational study identifies manometric abnormalities and pH testing in chronic alcoholics during withdrawal.
Twenty-four hours ambulatory esophageal manometry and pH-metry have also been used to study 23 chronic alcoholic subjects (median ethanol consumption 95 g/day for 12 years) and 12 control subjects in Sri Lanka (98). Autonomic nerve function tests were also performed. Autonomic neuropathy was observed in 43% (n=10) and although 34% reported reflux symptoms, GERD was confirmed with pH-metry in only 17% (n=4) (99). High LESPs were observed in those with autonomic neuropathy, as previously reported; in addition, alcoholic subjects, unlike controls, did not increase esophageal contraction amplitudes in the supine versus upright position, nor did they increase esophageal contractions post-prandially. Otherwise, esophageal body motility parameters were similar between alcoholic and control subjects (98). Differences in average total lifetime ethanol intake, exclusion of heavy smokers, and dietary factors may account for differences in findings between studies.
The effect of alcohol on esophageal motility has been further investigated in animal models. The cat has been used as an animal model to study the effect of ethanol on esophageal motor function (99) due to anatomic and physiologic similarities with the human esophagus and due to generally similar responses to ethanol. Cats were exposed to chronic ethanol exposure (dose of 300 mg/dL via a G-tube for 30 days) and studied in intoxicated and withdrawing states; observations generally mirrored those in humans, with elevations in LESP and smooth muscle esophageal contraction amplitudes in the withdrawing state in male cats (99). Increases in esophageal contractions’ amplitudes were observed in the upper portion of the esophagus (striated muscle) in the intoxicated and withdrawal states in male and female cats (99). Acute ethanol intoxication has also been studied in the cat model (100). Similar to what has been observed in humans, intravenous ethanol decreased LESP and the amplitude of esophageal smooth muscle contractions; these effects were less pronounced in cats subjected to chronic alcohol. In addition, the effects of ethanol were not inhibited by bilateral vagotomy or administration of the neurotoxin tetrodotoxin, suggesting effects of alcohol were mediated via direct effect on muscle (100). The utility of these animal models is that they allow for mechanistic studies that, for obvious reasons, cannot be readily performed in human subjects.
Alcohol and GERD
In humans, several studies show an increase in gastroesophageal reflux symptoms associated with alcohol intake. Multivariate analysis of a population-based study in Germany showed an odds ratio (OR) of 1.63 for drinking spirits several times a week (101). A community-based study in the UK showed excessive alcohol consumption (per an administered questionnaire defined as more than 30 units/week for men and more than 20 units/week for women) was also independently associated with GERD symptoms with an OR of 2.96 overall and 3.23 in men (102).
Alcohol consumption increases symptoms of reflux, likely by multiple mechanisms, including effects on salivary flow (103) and impaired esophageal motility (93-95). In healthy volunteers, 300 mL of a low proof alcoholic beverage such as white wine, induced gastroesophageal reflux characterized by prolonged duration, due to an increase in simultaneous esophageal contractions and a failure of peristalsis (104,105). In addition, the main mechanism of reflux appeared to be gastro-esophageal reflux, while the esophageal pH is still acidic from a prior episode (104,105). Dose and type of alcoholic beverage are also determinants of the amount of reflux induced (105).
In individuals with pre-existing non-erosive (normal endoscopy + pH metry study) and erosive (seen during endoscopy) GERD, randomized physiologic studies have also shown that consumption of 300 mL of white wine or 500 mL beer similarly induces gastroesophageal reflux with an increased esophageal percentage of time with pH <4 (106). Later studies in GERD patients suggested that the effects were strongest with white wine (105). Ultimately effects on the LES and GERD mechanisms may depend on the type of alcohol consumed, e.g., white versus red wine (107).
Regarding the association of alcohol consumption and the development of esophagitis, most earlier endoscopic studies showed a low percentage of esophagitis in alcoholic subjects (94,97,98). However, in an endoscopic study of chronic alcoholics who had been abstinent from alcohol for 2 weeks, but who had persistent upper gastrointestinal symptoms, visible esophagitis was the most common finding in 72% (56/78) (108). A large case control study from Ireland investigated the association between alcohol consumption and the development of reflux esophagitis (defined as Los Angeles Classification grades B, C, or D), as well as with BE, and EAC (109). Alcohol consumption was evaluated based on recall via interview and structured computerized interview at age 21 years and also at 5 years prior to the interview date. Consumption of alcohol (at least 1 alcoholic drink per month up to 39.7 drinks per week) in early adulthood (age 21 years), particularly beer, was associated with an increased risk of esophagitis (OR 2.24, 1.35–3.74) (109). When evaluating alcohol consumption 5 years prior to the study interview, interestingly, individuals with reflux esophagitis were more likely than controls (OR, 2.35; 95% CI: 1.30–4.27) to binge drink (defined as more than 7 alcoholic drinks in a day). In addition, high liquor intake (>4.5 drinks per week) was also associated with a 2-fold increased risk of reflux esophagitis (109). Alcohol consumption was not associated with an increased risk of BE (OR 1.06, 0.83–1.79) or EAC (OR 1.27, 0.77–2.10) (109). This along with other studies, does not support an association between BE or EAC and alcohol use (17,110).
Overall, the results of clinical studies investigating the association between alcohol consumption and GERD, have been somewhat variable and dependent on whether GERD symptoms are the outcome measure (usually determined by questionnaire) or whether objective evidence of GERD (esophagitis, BE, positive pH study) is under consideration (111). For example, a systematic review and meta-analysis of 102 community-based, population-based studies reported the prevalence of and risk factors for gastroesophageal reflux symptoms in adults using symptom-based criteria or questionnaires (112). Twenty-four studies reported on the prevalence of GERD symptoms in the setting of alcohol use; the pooled prevalence of GERD was slightly higher in alcohol drinkers compared to non-drinkers, but the summary odds ratio was non-significant 1.1, with substantial heterogeneity between the studies (112). A more recent systematic review and meta-analysis of 26 cross-sectional studies and 3 case-control studies investigated the relationship between GERD and alcohol consumption (grams of ethanol per day) (113). Esophagitis as diagnosed by upper endoscopy, was an outcome measure in 16 studies, symptoms were used as an outcome measure in 10 studies, and both esophagitis and symptoms were used as outcome measures in 3 studies (113). More than 2 quantitative categories of alcohol exposure were reported in 3 studies, allowing for dose-response analysis (113). Comparing all drinkers and non-/occasional drinkers, the pooled random effects OR was 1.48 (95% CI, 1.31–1.67; P<0.001), although the heterogeneity between studies was high (113). In subgroup analysis of erosive versus nonerosive GERD, again when comparing drinkers with non-/occasional drinkers, the pooled OR was higher for erosive esophagitis (OR 1.78; 95% CI, 1.56–2.03, low heterogeneity) than for non-erosive reflux disease (OR 1.15; 95% CI, 1.04–1.28, high heterogeneity) (113). When considering drinking frequency, the pooled OR for those who drank <3–5 times or days per week versus non-/occasional drinkers was 1.29 (95% CI, 1.14–1.46); while that for more frequent drinkers (3–5 times or days per week) was 2.23 (95% CI, 1.63–2.75) (113). Studies informing these results had moderate heterogeneity. Finally, dose-response analysis (based on the 3 studies that had more than two categories of alcohol exposure) showed a linear association between alcohol consumption and GERD (Pfor nonlinearity=0.235) with a pooled OR for a 12.5 g/day increment of alcohol of 1.16 (113). The provided dose-response analysis curve showed OR for up to 50 gm of alcohol intake a day, with a maximum OR of 1.8 (113). Differences in alcohol dose and frequency, grading of esophagitis, and comorbidities amongst studied populations may account for contradictory findings across studies.
Mechanisms by which alcohol consumption may lead to reflux esophagitis and not just GERD symptoms are not entirely known, but feasible possibilities include poor clearance of the refluxate due to impaired esophageal motility compounded by additional interference with the gastro-esophageal anti-reflux barrier via effects on the LES (114). As described above, animal studies also suggest that the deleterious effect of alcohol on epithelial tissue resistance (90,91) may also predispose the esophagus to acid-induced injury. In addition, stimulation of acid secretion has been reported with some forms of alcohol produced by fermentation, e.g., with beer and wine alcoholic beverages, leading to more acidic and therefore, damaging refluxate (115). The effects of pure ethanol and the various available distilled and fermented alcohol beverages on stimulation of gastric acid secretion in healthy humans are different; e.g., the effect of ethanol by itself is concentration dependent with 1.4% and 5% pure ethanol having a small stimulatory effect on gastric acid output (23% of maximal acid output) while higher concentrations including up to 40% had no effect or a mildly inhibitory effect (115).
Strengths and limitations
A strength of this narrative review is the comprehensive consideration of the effects of alcohol on the esophagus, not only based on epidemiologic studies, as well as what is known about the effects on the esophagus at cellular and physiologic levels. The risk of ESCC associated with alcohol consumption as well as associated mechanisms, has been extensively reviewed. Studies on the effect of alcohol on signaling in the esophagus, including changes in the esophageal microbiome, particularly prior to the development of malignancy, were also reviewed, as this knowledge could improve understanding of the pathogenesis of alcohol related diseases. However, a limitation of this review is that signaling studies that were considered were primarily informed by other tissues, as data in esophagus remains limited. Furthermore, studies focused on the esophagus that have been e.g., conducted in monocultures do not take into account complex interactions between varied cell types within the compartments of the esophageal mucosa (epithelium and lamina propria). 3D esophageal organoids, while a marked advancement, also imperfectly recapitulate the esophageal mucosa. These models, however, particularly when established by human-derived tissue, currently remain the best available tools for in vitro studies in the esophagus.
An additional strength of the review is the discussion of physiological studies related to alcohol as well as observational/epidemiologic studies related to one of the most common esophageal disorders, GERD. As expected, it remains difficult to conduct pathophysiologic studies with alcohol without a dedicated physiology lab and with expected difficulty in patient enrollment in these types of studies. A number of physiological studies investigating the effect of alcohol consumption on normal healthy volunteers, in alcoholics and in animal models of ethanol-induced esophageal motor dysfunction have been reviewed. However, the study designs were heterogeneous across studies including differences in dose and/or duration of administered alcohol in quantification of alcohol consumption and did not include molecular analysis. Similarly, there is a wide range of alcohol concentrations and durations that have been utilized in the reviewed in vitro studies. In vitro studies and even controlled in vivo studies with humans may also not reflect how humans drink in “real-life” situations. In vitro modeling that would better reflect or mimic the varied ways of human alcohol consumption and that would also lend itself to mechanistic studies remains lacking. Additional investigation of the molecular effects of alcohol on the human esophagus is warranted, including e.g., via carefully designed high throughout put sequencing performed on in vitro models that mimic “real-life” alcohol intake.
Conclusions
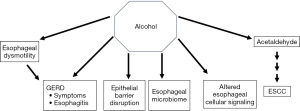
Alcohol exerts a multitude of effects in the esophagus, via direct effects and its toxic metabolite acetaldehyde (Figure 1). The strongest association by far is with ESCC. Although a threshold level at which risk significantly increases is not well defined, it is clear that higher alcohol consumption increases risk, particularly in individuals with genetic variants in the enzymes responsible for ethanol metabolism. Studies also suggest a role for alcohol consumption in observed alterations in the esophageal microbiome, in signaling at the cellular level, as well as in esophageal motility. Alcohol consumption has also been associated with reflux symptoms and less so with reflux esophagitis. Available studies do not support its role as a risk factor for BE or EAC. In an era of increasing obesity and parallel increases in GERD, however, it is conceivable that the impaired epithelial barrier associated with these two particular conditions can allow for penetration of toxic luminal agents such as alcohol beyond the squamous epithelium. Interestingly, the neo-squamous epithelium of treated BE is also characterized by defective barrier function (13). As such, the effect of alcohol on modulating cellular behavior in these cases deserves attention.
Over the past few decades, there has been a startling increase in the prevalence and incidence of eosinophilic esophagitis, another esophageal disorder characterized by an impaired epithelial barrier, which will allow for penetration of toxic agents. A recent population-based, nested case-control study showed alcohol consumption was associated with increased risk of eosinophilic esophagitis (EoE) (OR 1.51, 1.21–1.88, P<0.001) (116). In this study, alcohol consumption was determined from retrospective chart review of the most recent clinical visit and defined as “always” or “sometimes”, without additional clarification. On the other hand, a case-control study analyzing data of a prospectively collected adult cohort from the University of North Carolina showed that although alcohol use was more common among those with EoE (75% versus 51%, P<0.001), multivariate analysis did not show an association. In other words, although alcohol use was more prevalent, it was not identified as an independent risk factor (117). Differences in study design and population may account for the discrepancy in findings between the two studies. Future studies should address whether epithelial barrier disruption due to alcohol consumption plays a role in the development of EoE or the response to existing therapies.
Regardless, given the increasing prevalence of esophageal disorders with epithelial barrier disruption, the effect of alcohol, an agent that continues to be used and misused, should continue to be investigated. Optimization of in vitro models is needed, e.g., via the use of 3D organoids using primary human tissue and along with carefully designed high throughout put sequencing studies, should be leveraged to better study the effect of alcohol in normal and diseased esophagus. In the meantime, increased awareness of the established and potential risks of alcohol consumption will better inform our patients and help in their personal decision whether to cease or, at very least, moderate their alcohol consumption.
Acknowledgments
None.
Footnote
Reporting Checklist: The author has completed the Narrative Review reporting checklist. Available at https://aoe.amegroups.com/article/view/10.21037/aoe-24-46/rc
Peer Review File: Available at https://aoe.amegroups.com/article/view/10.21037/aoe-24-46/prf
Funding: This work was supported by
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://aoe.amegroups.com/article/view/10.21037/aoe-24-46/coif). This work was supported by the National Institutes of Health (No. AA028891). The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Manthey J, Shield KD, Rylett M, et al. Global alcohol exposure between 1990 and 2017 and forecasts until 2030: a modelling study. Lancet 2019;393:2493-502. [Crossref] [PubMed]
- Rehm J, Probst C. Decreases of Life Expectancy Despite Decreases in Non-Communicable Disease Mortality: The Role of Substance Use and Socioeconomic Status. Eur Addict Res 2018;24:53-9. [Crossref] [PubMed]
- Martinez P, Kerr WC, Subbaraman MS, et al. New Estimates of the Mean Ethanol Content of Beer, Wine, and Spirits Sold in the United States Show a Greater Increase in Per Capita Alcohol Consumption than Previous Estimates. Alcohol Clin Exp Res 2019;43:509-21. [Crossref] [PubMed]
- Fisher RS, Malmud LS, Applegate G, et al. Effect of bolus composition on esophageal transit: concise communication. J Nucl Med 1982;23:878-82. [PubMed]
- Dolganiuc A, Szabo G. In vitro and in vivo models of acute alcohol exposure. World J Gastroenterol 2009;15:1168-77. [Crossref] [PubMed]
- Liu Y, Chen H, Sun Z, et al. Molecular mechanisms of ethanol-associated oro-esophageal squamous cell carcinoma. Cancer Lett 2015;361:164-73. [Crossref] [PubMed]
- Salaspuro M. Local Acetaldehyde: Its Key Role in Alcohol-Related Oropharyngeal Cancer. Visc Med 2020;36:167-73. [Crossref] [PubMed]
- Oyama T, Isse T, Kagawa N, et al. Tissue-distribution of aldehyde dehydrogenase 2 and effects of the ALDH2 gene-disruption on the expression of enzymes involved in alcohol metabolism. Front Biosci 2005;10:951-60. [Crossref] [PubMed]
- Seitz HK, Stickel F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat Rev Cancer 2007;7:599-612. [Crossref] [PubMed]
- O’Shea KM, Aceves SS, Dellon ES, et al. Pathophysiology of Eosinophilic Esophagitis. Gastroenterology 2018;154:333-45. [Crossref] [PubMed]
- Tack J, Pandolfino JE. Pathophysiology of Gastroesophageal Reflux Disease. Gastroenterology 2018;154:277-88. [Crossref] [PubMed]
- Gibbens YY, Lansing R, Johnson ML, et al. Effects of Central Obesity on Esophageal Epithelial Barrier Function. Am J Gastroenterol 2021;116:1537-41. [Crossref] [PubMed]
- Jovov B, Shaheen NJ, Orlando GS, et al. Defective barrier function in neosquamous epithelium. Am J Gastroenterol 2013;108:386-91. [Crossref] [PubMed]
- Abnet CC, Arnold M, Wei WQ. Epidemiology of Esophageal Squamous Cell Carcinoma. Gastroenterology 2018;154:360-73. [Crossref] [PubMed]
- Vanella G, Archibugi L, Stigliano S, et al. Alcohol and gastrointestinal cancers. Curr Opin Gastroenterol 2019;35:107-13. [Crossref] [PubMed]
- Freedman ND, Murray LJ, Kamangar F, et al. Alcohol intake and risk of oesophageal adenocarcinoma: a pooled analysis from the BEACON Consortium. Gut 2011;60:1029-37. [Crossref] [PubMed]
- Thrift AP, Cook MB, Vaughan TL, et al. Alcohol and the risk of Barrett’s esophagus: a pooled analysis from the International BEACON Consortium. Am J Gastroenterol 2014;109:1586-94. [Crossref] [PubMed]
- Coleman HG, Xie SH, Lagergren J. The Epidemiology of Esophageal Adenocarcinoma. Gastroenterology 2018;154:390-405. [Crossref] [PubMed]
- Cook MB, Thrift AP. Epidemiology of Barrett’s Esophagus and Esophageal Adenocarcinoma: Implications for Screening and Surveillance. Gastrointest Endosc Clin N Am 2021;31:1-26. [Crossref] [PubMed]
- Freedman ND, Abnet CC, Leitzmann MF, et al. A prospective study of tobacco, alcohol, and the risk of esophageal and gastric cancer subtypes. Am J Epidemiol 2007;165:1424-33. [Crossref] [PubMed]
- Steevens J, Schouten LJ, Goldbohm RA, et al. Alcohol consumption, cigarette smoking and risk of subtypes of oesophageal and gastric cancer: a prospective cohort study. Gut 2010;59:39-48. [Crossref] [PubMed]
- Bagnardi V, Blangiardo M, La Vecchia C, et al. A meta-analysis of alcohol drinking and cancer risk. Br J Cancer 2001;85:1700-5. [Crossref] [PubMed]
- Bagnardi V, Rota M, Botteri E, et al. Light alcohol drinking and cancer: a meta-analysis. Ann Oncol 2013;24:301-8. [Crossref] [PubMed]
- Castellsagué X, Muñoz N, De Stefani E, et al. Independent and joint effects of tobacco smoking and alcohol drinking on the risk of esophageal cancer in men and women. Int J Cancer 1999;82:657-64. [Crossref] [PubMed]
- Lubin JH, Cook MB, Pandeya N, et al. The importance of exposure rate on odds ratios by cigarette smoking and alcohol consumption for esophageal adenocarcinoma and squamous cell carcinoma in the Barrett’s Esophagus and Esophageal Adenocarcinoma Consortium. Cancer Epidemiol 2012;36:306-16. [Crossref] [PubMed]
- Fahey PP, Mallitt KA, Astell-Burt T, et al. Impact of pre-diagnosis behavior on risk of death from esophageal cancer: a systematic review and meta-analysis. Cancer Causes Control 2015;26:1365-73. [Crossref] [PubMed]
- Bagnardi V, Rota M, Botteri E, et al. Alcohol consumption and site-specific cancer risk: a comprehensive dose-response meta-analysis. Br J Cancer 2015;112:580-93. [Crossref] [PubMed]
- Castro C, Peleteiro B, Lunet N. Modifiable factors and esophageal cancer: a systematic review of published meta-analyses. J Gastroenterol 2018;53:37-51. [Crossref] [PubMed]
- Yu X, Chen J, Jiang W, et al. Alcohol, Alcoholic Beverages and Risk of Esophageal Cancer by Histological Type: A Dose-Response Meta-Analysis of Observational Studies. Alcohol Alcohol 2020;55:457-67. [Crossref] [PubMed]
- Dufour MC. What is moderate drinking? Defining "drinks" and drinking levels. Alcohol Res Health 1999;23:5-14. [PubMed]
- Prabhu A, Obi KO, Rubenstein JH. Systematic review with meta-analysis: race-specific effects of alcohol and tobacco on the risk of oesophageal squamous cell carcinoma. Aliment Pharmacol Ther 2013;38:1145-55. [Crossref] [PubMed]
- Rota M, Bellocco R, Scotti L, et al. Random-effects meta-regression models for studying nonlinear dose-response relationship, with an application to alcohol and esophageal squamous cell carcinoma. Stat Med 2010;29:2679-87. [Crossref] [PubMed]
- Pöschl G, Seitz HK. Alcohol and cancer. Alcohol Alcohol 2004;39:155-65. [Crossref] [PubMed]
- Alcohol consumption and ethyl carbamate. IARC Monogr Eval Carcinog Risks Hum 2010;96:3-1383. [PubMed]
- Ohashi S, Miyamoto S, Kikuchi O, et al. Recent Advances From Basic and Clinical Studies of Esophageal Squamous Cell Carcinoma. Gastroenterology 2015;149:1700-15. [Crossref] [PubMed]
- Molina JC, Guerrero-Morán JD, González-Espinosa C. Alcohol: Immunomodulatory Effects and Cancer. Rev Invest Clin 2023;75:129-42. [Crossref] [PubMed]
- Liu K, Song G, Zhu X, et al. Association between ALDH2 Glu487Lys polymorphism and the risk of esophageal cancer. Medicine (Baltimore) 2017;96:e6111. [Crossref] [PubMed]
- Yang SJ, Yokoyama A, Yokoyama T, et al. Relationship between genetic polymorphisms of ALDH2 and ADH1B and esophageal cancer risk: a meta-analysis. World J Gastroenterol 2010;16:4210-20. [Crossref] [PubMed]
- Morita M, Oyama T, Kagawa N, et al. Expression of aldehyde dehydrogenase 2 in the normal esophageal epithelium and alcohol consumption in patients with esophageal cancer. Front Biosci 2005;10:2319-24. [Crossref] [PubMed]
- Amanuma Y, Ohashi S, Itatani Y, et al. Protective role of ALDH2 against acetaldehyde-derived DNA damage in oesophageal squamous epithelium. Sci Rep 2015;5:14142. [Crossref] [PubMed]
- Rossini A, Rapozo DC, Soares Lima SC, et al. Polymorphisms of GSTP1 and GSTT1, but not of CYP2A6, CYP2E1 or GSTM1, modify the risk for esophageal cancer in a western population. Carcinogenesis 2007;28:2537-42. [Crossref] [PubMed]
- Di Sarno R, Brigida A, Caprio GG, et al. Critical review on the use and abuse of alcohol. When the dose makes the difference. Minerva Med 2020;111:344-53. [Crossref] [PubMed]
- Peng Q, Chen H, Huo JR. Alcohol consumption and corresponding factors: A novel perspective on the risk factors of esophageal cancer. Oncol Lett 2016;11:3231-9. [Crossref] [PubMed]
- May M, Abrams JA. Emerging Insights into the Esophageal Microbiome. Curr Treat Options Gastroenterol 2018;16:72-85. [Crossref] [PubMed]
- Park CH, Lee SK. Exploring Esophageal Microbiomes in Esophageal Diseases: A Systematic Review. J Neurogastroenterol Motil 2020;26:171-9. [Crossref] [PubMed]
- Chiang HC, Hughes M, Chang WL. The role of microbiota in esophageal squamous cell carcinoma: A review of the literature. Thorac Cancer 2023;14:2821-9. [Crossref] [PubMed]
- Fan X, Peters BA, Jacobs EJ, et al. Drinking alcohol is associated with variation in the human oral microbiome in a large study of American adults. Microbiome 2018;6:59. [Crossref] [PubMed]
- Radani N, Metwaly A, Reitmeier S, et al. Analysis of Fecal, Salivary, and Tissue Microbiome in Barrett’s Esophagus, Dysplasia, and Esophageal Adenocarcinoma. Gastro Hep Adv 2022;1:755-66. [Crossref] [PubMed]
- Li Z, Liu Y, Dou L, et al. The effects of smoking and drinking on the oral and esophageal microbiota of healthy people. Ann Transl Med 2021;9:1244. [Crossref] [PubMed]
- O’Grady I, Anderson A, O’Sullivan J. The interplay of the oral microbiome and alcohol consumption in oral squamous cell carcinomas. Oral Oncol 2020;110:105011. [Crossref] [PubMed]
- Chen X, Winckler B, Lu M, et al. Oral Microbiota and Risk for Esophageal Squamous Cell Carcinoma in a High-Risk Area of China. PLoS One 2015;10:e0143603. [Crossref] [PubMed]
- Yu G, Gail MH, Shi J, et al. Association between upper digestive tract microbiota and cancer-predisposing states in the esophagus and stomach. Cancer Epidemiol Biomarkers Prev 2014;23:735-41. [Crossref] [PubMed]
- Rao W, Lin Z, Liu S, et al. Association between alcohol consumption and oesophageal microbiota in oesophageal squamous cell carcinoma. BMC Microbiol 2021;21:73. [Crossref] [PubMed]
- Nath P, Anand AC. Extrahepatic Manifestations in Alcoholic Liver Disease. J Clin Exp Hepatol 2022;12:1371-83. [Crossref] [PubMed]
- Szabo G, Dolganiuc A, Dai Q, et al. TLR4, ethanol, and lipid rafts: a new mechanism of ethanol action with implications for other receptor-mediated effects. J Immunol 2007;178:1243-9. [Crossref] [PubMed]
- Oak S, Mandrekar P, Catalano D, et al. TLR2- and TLR4-mediated signals determine attenuation or augmentation of inflammation by acute alcohol in monocytes. J Immunol 2006;176:7628-35. [Crossref] [PubMed]
- Sheyhidin I, Nabi G, Hasim A, et al. Overexpression of TLR3, TLR4, TLR7 and TLR9 in esophageal squamous cell carcinoma. World J Gastroenterol 2011;17:3745-51. [Crossref] [PubMed]
- Carreras-Gallo N, Dwaraka VB, Cáceres A, et al. Impact of tobacco, alcohol, and marijuana on genome-wide DNA methylation and its relationship with hypertension. Epigenetics 2023;18:2214392. [Crossref] [PubMed]
- Rumgay H, Murphy N, Ferrari P, et al. Alcohol and Cancer: Epidemiology and Biological Mechanisms. Nutrients 2021;13:3173. [Crossref] [PubMed]
- Khalid O, Kim JJ, Kim HS, et al. Gene expression signatures affected by alcohol-induced DNA methylomic deregulation in human embryonic stem cells. Stem Cell Res 2014;12:791-806. [Crossref] [PubMed]
- Lohoff FW, Clarke TK, Kaminsky ZA, et al. Epigenome-wide association study of alcohol consumption in N = 8161 individuals and relevance to alcohol use disorder pathophysiology: identification of the cystine/glutamate transporter SLC7A11 as a top target. Mol Psychiatry 2022;27:1754-64. [Crossref] [PubMed]
- Yan Y, Teng H, Hang Q, et al. SLC7A11 expression level dictates differential responses to oxidative stress in cancer cells. Nat Commun 2023;14:3673. [Crossref] [PubMed]
- Xu Y, Wang Z, Pei B, et al. DNA methylation markers in esophageal cancer. Front Genet 2024;15:1354195. [Crossref] [PubMed]
- Gerber JK, Richter T, Kremmer E, et al. Progressive loss of PAX9 expression correlates with increasing malignancy of dysplastic and cancerous epithelium of the human oesophagus. J Pathol 2002;197:293-7. [Crossref] [PubMed]
- Xiong Z, Ren S, Chen H, et al. PAX9 regulates squamous cell differentiation and carcinogenesis in the oro-oesophageal epithelium. J Pathol 2018;244:164-75. [Crossref] [PubMed]
- Shi M, Ren S, Chen H, et al. Alcohol drinking inhibits NOTCH-PAX9 signaling in esophageal squamous epithelial cells. J Pathol 2021;253:384-95. [Crossref] [PubMed]
- Li Y, Li Y, Chen X. NOTCH and Esophageal Squamous Cell Carcinoma. Adv Exp Med Biol 2021;1287:59-68. [Crossref] [PubMed]
- Kasagi Y, Chandramouleeswaran PM, Whelan KA, et al. The Esophageal Organoid System Reveals Functional Interplay Between Notch and Cytokines in Reactive Epithelial Changes. Cell Mol Gastroenterol Hepatol 2018;5:333-52. [Crossref] [PubMed]
- Li YX, Yang HT, Zdanowicz M, et al. Fetal alcohol exposure impairs Hedgehog cholesterol modification and signaling. Lab Invest 2007;87:231-40. [Crossref] [PubMed]
- Mao H, Diehl AM, Li YX. Sonic hedgehog ligand partners with caveolin-1 for intracellular transport. Lab Invest 2009;89:290-300. [Crossref] [PubMed]
- McCarthy N, Eberhart JK. Gene-ethanol interactions underlying fetal alcohol spectrum disorders. Cell Mol Life Sci 2014;71:2699-706. [Crossref] [PubMed]
- Ng CK, Ma K, Cheng Y, et al. Kruppel-like Factor 5 Promotes Sonic Hedgehog Signaling and Neoplasia in Barrett’s Esophagus and Esophageal Adenocarcinoma. Transl Oncol 2019;12:1432-41. [Crossref] [PubMed]
- Li L, Jiang D, Zhang Q, et al. Integrative proteogenomic characterization of early esophageal cancer. Nat Commun 2023;14:1666. [Crossref] [PubMed]
- Moody S, Senkin S, Islam SMA, et al. Mutational signatures in esophageal squamous cell carcinoma from eight countries with varying incidence. Nat Genet 2021;53:1553-63. [Crossref] [PubMed]
- Hoes L, Voordeckers K, Dok R, et al. Ethanol induces replication fork stalling and membrane stress in immortalized laryngeal cells. iScience 2023;26:108564. [Crossref] [PubMed]
- Luo J, Chen XQ, Li P. The Role of TGF-beta and Its Receptors in Gastrointestinal Cancers. Transl Oncol 2019;12:475-84. [Crossref] [PubMed]
- Lurje I, Gaisa NT, Weiskirchen R, et al. Mechanisms of organ fibrosis: Emerging concepts and implications for novel treatment strategies. Mol Aspects Med 2023;92:101191. [Crossref] [PubMed]
- Muir AB, Karakasheva TA, Whelan KA. Epithelial-Fibroblast Crosstalk in Eosinophilic Esophagitis. Cell Mol Gastroenterol Hepatol 2024;17:713-8. [Crossref] [PubMed]
- Gu S, Nguyen BN, Rao S, et al. Alcohol, stem cells and cancer. Genes Cancer 2017;8:695-700. [Crossref] [PubMed]
- Law BA, Carver WE. Activation of cardiac fibroblasts by ethanol is blocked by TGF-beta inhibition. Alcohol Clin Exp Res 2013;37:1286-94. [Crossref] [PubMed]
- Khalatbari A, Castle JD, Li T, et al. Direct and indirect effects of alcohol and its toxic metabolite acetaldehyde on human esophageal myofibroblasts and epithelial cells. Alcohol Clin Exp Res (Hoboken) 2023;47:1297-311. [Crossref] [PubMed]
- Kornfehl J, Temmel A, Formanek M, et al. Effects of ethanol treatment of proliferation and differentiation in a head and neck squamous cell carcinoma cell line. Alcohol Clin Exp Res 1999;23:1102-7. [Crossref] [PubMed]
- Chandramouleeswaran PM, Guha M, Shimonosono M, et al. Autophagy mitigates ethanol-induced mitochondrial dysfunction and oxidative stress in esophageal keratinocytes. PLoS One 2020;15:e0239625. [Crossref] [PubMed]
- Zhang M, Ma Y, Ye X, et al. TRP (transient receptor potential) ion channel family: structures, biological functions and therapeutic interventions for diseases. Signal Transduct Target Ther 2023;8:261. [Crossref] [PubMed]
- Gargus M, Niu C, Vallone JG, et al. Human esophageal myofibroblasts secrete proinflammatory cytokines in response to acid and Toll-like receptor 4 ligands. Am J Physiol Gastrointest Liver Physiol 2015;308:G904-23. [Crossref] [PubMed]
- Trevisani M, Smart D, Gunthorpe MJ, et al. Ethanol elicits and potentiates nociceptor responses via the vanilloid receptor-1. Nat Neurosci 2002;5:546-51. [Crossref] [PubMed]
- Monteith GR, McAndrew D, Faddy HM, et al. Calcium and cancer: targeting Ca2+ transport. Nat Rev Cancer 2007;7:519-30. [Crossref] [PubMed]
- Crotty K, Anton P, Coleman LG, et al. A critical review of recent knowledge of alcohol’s effects on the immunological response in different tissues. Alcohol Clin Exp Res (Hoboken) 2023;47:36-44. [Crossref] [PubMed]
- Bor S, Caymaz-Bor C, Tobey NA, et al. Effect of ethanol on the structure and function of rabbit esophageal epithelium. Am J Physiol 1998;274:G819-26. [PubMed]
- Bor S, Bor-Caymaz C, Tobey NA, et al. Esophageal exposure to ethanol increases risk of acid damage in rabbit esophagus. Dig Dis Sci 1999;44:290-300. [Crossref] [PubMed]
- Bor S, Capanoglu D. The additive effect of ethanol and extract of cigarette smoke on rabbit esophagus epithelium. J Gastroenterol Hepatol 2009;24:316-21. [Crossref] [PubMed]
- Rubinstein E, Hauge C, Sommer P, et al. Oesophageal and gastric potential difference and pH in healthy volunteers following intake of coca-cola, red wine, and alcohol. Pharmacol Toxicol 1993;72:61-5. [Crossref] [PubMed]
- Hogan WJ, Viegas de Andrade SR, Winship DH. Ethanol-induced acute esophageal motor dysfunction. J Appl Physiol 1972;32:755-60. [Crossref] [PubMed]
- Keshavarzian A, Iber FL, Ferguson Y. Esophageal manometry and radionuclide emptying in chronic alcoholics. Gastroenterology 1987;92:651-7. [Crossref] [PubMed]
- Keshavarzian A, Polepalle C, Iber FL, et al. Esophageal motor disorder in alcoholics: result of alcoholism or withdrawal? Alcohol Clin Exp Res 1990;14:561-7. [Crossref] [PubMed]
- Keshavarzian A, Polepalle C, Iber FL, et al. Secondary esophageal contractions are abnormal in chronic alcoholics. Dig Dis Sci 1992;37:517-22. [Crossref] [PubMed]
- Grande L, Monforte R, Ros E, et al. High amplitude contractions in the middle third of the oesophagus: a manometric marker of chronic alcoholism? Gut 1996;38:655-62. [Crossref] [PubMed]
- Ferdinandis TG, Dissanayake AS, de Silva HJ. Chronic alcoholism and esophageal motor activity: a 24-h ambulatory manometry study. J Gastroenterol Hepatol 2006;21:1157-62. [Crossref] [PubMed]
- Keshavarzian A, Rizk G, Urban G, et al. Ethanol-induced esophageal motor disorder: development of an animal model. Alcohol Clin Exp Res 1990;14:76-81. [Crossref] [PubMed]
- Keshavarzian A, Urban G, Sedghi S, et al. Effect of acute ethanol on esophageal motility in cat. Alcohol Clin Exp Res 1991;15:116-21. [Crossref] [PubMed]
- Nocon M, Labenz J, Jaspersen D, et al. Health-related quality of life in patients with gastro-oesophageal reflux disease under routine care: 5-year follow-up results of the ProGERD study. Aliment Pharmacol Ther 2009;29:662-8. [Crossref] [PubMed]
- Mohammed I, Nightingale P, Trudgill NJ. Risk factors for gastro-oesophageal reflux disease symptoms: a community study. Aliment Pharmacol Ther 2005;21:821-7. [Crossref] [PubMed]
- Reidy J, McHugh E, Stassen LF. A review of the relationship between alcohol and oral cancer. Surgeon 2011;9:278-83. [Crossref] [PubMed]
- Pehl C, Frommherz M, Wendl B, et al. Gastroesophageal reflux induced by white wine: the role of acid clearance and "rereflux". Am J Gastroenterol 2002;97:561-7. [Crossref] [PubMed]
- Seidl H, Gundling F, Schepp W, et al. Effect of low-proof alcoholic beverages on duodenogastro-esophageal reflux in health and GERD. Neurogastroenterol Motil 2011;23:145-50, e29.
- Pehl C, Wendl B, Pfeiffer A. White wine and beer induce gastro-oesophageal reflux in patients with reflux disease. Aliment Pharmacol Ther 2006;23:1581-6. [Crossref] [PubMed]
- Pehl C, Pfeiffer A, Wendl B, et al. Different effects of white and red wine on lower esophageal sphincter pressure and gastroesophageal reflux. Scand J Gastroenterol 1998;33:118-22. [Crossref] [PubMed]
- Tønnesen H, Andersen JR, Christoffersen P, et al. Reflux oesophagitis in heavy drinkers. Effect of ranitidine and alginate/metoclopramide. Digestion 1987;38:69-73. [Crossref] [PubMed]
- Anderson LA, Cantwell MM, Watson RG, et al. The association between alcohol and reflux esophagitis, Barrett’s esophagus, and esophageal adenocarcinoma. Gastroenterology 2009;136:799-805. [Crossref] [PubMed]
- Thrift AP. Barrett’s Esophagus and Esophageal Adenocarcinoma: How Common Are They Really? Dig Dis Sci 2018;63:1988-96. [Crossref] [PubMed]
- Richter JE, Rubenstein JH. Presentation and Epidemiology of Gastroesophageal Reflux Disease. Gastroenterology 2018;154:267-76. [Crossref] [PubMed]
- Eusebi LH, Ratnakumaran R, Yuan Y, et al. Global prevalence of, and risk factors for, gastro-oesophageal reflux symptoms: a meta-analysis. Gut 2018;67:430-40. [Crossref] [PubMed]
- Pan J, Cen L, Chen W, et al. Alcohol Consumption and the Risk of Gastroesophageal Reflux Disease: A Systematic Review and Meta-analysis. Alcohol Alcohol 2019;54:62-9. [Crossref] [PubMed]
- Argüero J, Sifrim D. Pathophysiology of gastro-oesophageal reflux disease: implications for diagnosis and management. Nat Rev Gastroenterol Hepatol 2024;21:282-93. [Crossref] [PubMed]
- Teyssen S, Singer MV. Alcohol-related diseases of the oesophagus and stomach. Best Pract Res Clin Gastroenterol 2003;17:557-73. [Crossref] [PubMed]
- Sawada A, Imai T, Ihara Y, et al. Epidemiology and Risk Factors of Eosinophilic Esophagitis in Japan: A Population-Based Study. Clin Gastroenterol Hepatol 2024;22:2023-2032.e6. [Crossref] [PubMed]
- Koutlas NT, Eluri S, Rusin S, et al. Impact of smoking, alcohol consumption, and NSAID use on risk for and phenotypes of eosinophilic esophagitis. Dis Esophagus 2018;31:1-7. [Crossref] [PubMed]
Cite this article as: Shaker A. Physiologic and molecular effects of alcohol in the esophagus: a narrative review. Ann Esophagus 2025;8:10.


