Endoluminal vacuum therapy using a fenestrated surgical drain for management of anastomotic leak following esophagectomy
Highlight box
Key findings
• 90% of patients treated with endoluminal vacuum therapy (EVT) using a drain experienced complete healing of the anastomotic leak (AL).
• There was 0% 90-day mortality with no serious adverse events related to EVT use.
• The majority (56%) of EVT with drain repositioning procedures were performed without general anaesthesia (GA), and there was a median duration of 7 days between EVT-related procedure and overall median duration of EVT therapy was 19.5 days (range, 5–72 days).
• These findings show comparable efficacy to studies reporting on the use of conventional EVT approaches using sponge-based devices. Additionally, EVT with drain provides several benefits including reduced material cost, reducing the need for frequent endoscopic device exchanges under GA and may be technically easier for an endoscopist to perform.
What is known and what is new?
• AL after esophagectomy is a major complication with increased risk of morbidity and mortality.
• EVT conventionally uses an endosponge with negative pressure suction and has proven effective in managing AL.
• This study introduces a novel approach, using a simple fenestrated surgical wound drain with negative pressure suction for EVT for management of AL.
What is the implication, and what should change now?
• EVT with drain appears to be a safe and effective technique for managing AL after esophagectomy. Further research is needed for direct comparisons between EVT with drain and other EVT approaches to determine the optimal management strategy for AL post-esophagectomy.
• A standardised reporting framework for EVT procedures is needed to identify optimal suction pressures with protocols and indications for its use.
Introduction
Background
Anastomotic leak (AL) occurs in 5–30% of esophagogastric resections and represents a major complication which may be associated with significant morbidity and mortality (1,2). The Esophagectomy Complications Consensus Group (ECCG) define AL as a ‘full thickness gastrointestinal defect involving esophagus, anastomosis, staple line, or conduit irrespective of presentation or method of identification’ (3). AL can be directly linked to mortality through sepsis but is also associated with reduced rates of adjuvant chemotherapy which may have a detrimental effect on survival (4). Management of post-esophagectomy AL can be further divided into three categories: ‘Type I leaks require no change in therapy, are treated medically or with dietary modification; type II leaks require interventional but not surgical therapy (interventional radiology drain, stent, etc.); type III leaks require surgical intervention’ (3).
Endoluminal vacuum therapy (EVT) for managing AL is gaining widespread popularity. Other endoscopic options have a limited role but include self-expanding metal stents (SEMS); clipping; fibrin glue injection and endoscopic suturing (5). With the advent of EVT there is a growing body of evidence to support its use. A recent meta-analysis and systematic review of EVT identified a successful healing rate of 81.6%, with 16% superiority to SEMS and comparably lower mortality rates (6). However, techniques are not standardised and the term EVT is heterogenous with respect to the different methods that can be used. In general, this would involve use of an ‘endosponge’, which remains the most common method and may involve the use of a commercial device such as Eso-SpongeÒ (B. Braun, Melsungen, Germany) or self-made adaptations (7,8). Another promising novel alternative device is the VACStentTM (MICRO-TECH Europe GmbH, Düsseldorf, Germany) which combines the effect of EVT and intraluminal stenting and has shown to be effective for both benign esophageal perforations and AL after esophagectomy (9,10). Additionally, it may have potential to be used pre-emptively to prevent AL after esophagectomy in high-risk cases (11).
Rationale and knowledge gap
Endosponge devices with applied negative pressure suction aim to promote healing through removal of necrotic debris and pus, thereby limiting further contamination and promoting tissue granulation and reducing interstitial oedema (12). Once an endosponge has been placed, the device will typically need to be replaced approximately every three to five days following insertion as the sponge would otherwise risk fixation to the cavity wall (7). Furthermore, most that use the endosponge technique advocate the use of general anaesthesia (GA) or deep sedation with propofol to replace the device (13,14). The combination of frequent device change and the need for GA is not only labour intensive but is also resource-dependent on availability of operating lists to accommodate the procedure. The ideal EVT device would have low material cost, reduce the need for frequent endoscopic exchanges under GA and be effective at promoting healing of the anastomotic defect. EVT with a fenestrated surgical wound drain has potential to address the limitations of endosponge-based approaches to management of AL. The benefits of this approach compared to alternative AL management strategies are numerous including reducing the need for frequent endoscopic device exchanges under GA, reduced material cost given the same drain can be used for the duration of therapy and ubiquitous availability of the required equipment. Additionally, it may be technically easier to perform as the drain does not become fixed to surrounding tissues as with comparable endosponge approaches. EVT using a surgical drain has not been described in the published literature. However, authors have described using a similar technique using a nasogastric sump drainage tube applied to vacuum suction for management of AL after Esophagectomy (15,16). The surgical drain used for the purpose of EVT is different from nasogastric tubes in that it has more fenestrations which have smaller diameters and an open tipped lumen. The safety and efficacy of this approach in management of AL requires characterisation given its promising potential for management of this major complication.
Objective
We describe our experience of a novel approach to EVT management of post-esophagectomy AL, using an endoscopically placed fenestrated surgical wound drain into the site of or adjacent to the anastomotic defect and applied to vacuum suction. We aimed to see whether EVT with a surgical drain was a technically feasible, safe, and efficacious approach to management of AL after esophagectomy that reduced the need for frequent GA-based exchange. We present this article in accordance with the STROBE reporting checklist (available at https://aoe.amegroups.org/article/view/10.21037/aoe-23-24/rc).
Methods
Study design
This single-centre, retrospective observational study was performed between August 2019 to March 2023. All operations were performed at the Norfolk and Norwich University Hospital which is an established high-volume tertiary esophagogastric cancer centre in the United Kingdom. The index operation and subsequent complications were managed by four consultant esophagogastric surgeons. Esophageal resections included Ivor-Lewis two stage procedures and McKeown three-stage procedures performed either as hybrid (laparoscopic abdomen and right-sided thoracotomy) or minimally invasively (laparoscopic abdomen and thoracoscopic chest) for esophageal cancer. Anastomoses were either stapled 25 or 29 mm ECHELON CIRCULAR™ Powered Stapler [Ethicon Endo-Surgery (Europe) GmbH, Norderstedt, Germany] or single layer interrupted hand sewn with the choice of technique dependent on surgeon preference. AL was detected through a combination of intravenous contrast enhanced computer tomography (CT), esophagogastroduodenoscopy (EGD) and water-soluble contrast swallow assessments. All patients with a type II AL grade, as defined by the ECCG, who were managed primarily with a fenestrated surgical drain inserted into the anastomotic defect cavity or an intraluminal location adjacent to the site of AL and applied to vacuum suction were included. Patients with type III leaks; presenting with florid mediastinitis, who initially needed emergency thoracotomy, washout and either placement of T-tube or anastomotic revision were excluded from this EVT series. Patients with type I leaks and type II leaks that were managed with an alternative strategy (e.g., esophageal stenting, endosponge) were also excluded.
Data collection
Patients were identified through the NorSTRA Upper Gastro-Intestinal Cancer database, which is a prospectively maintained database of patients undergoing Esophagogastric cancer resections at Norfolk and Norwich University Hospital. NorSTRA database maintains routinely collected clinical data fields including: (I) patient demographics, (II) indication for procedure, (III) type of surgery, (IV) neoadjuvant therapy, (V) operative details, (VI) post-operative complications and outcomes and (VII) histopathological outcomes. Additional data fields requiring (VIII) endoscopic and imaging findings were retrospectively extracted from patient electronic health care records. All patients included provided written informed consent for this technique and all other procedures associated with their hospital admission, and for the data to be anonymously collected and used for research purposes.
Evaluated variables and statistical analysis
The primary outcome assessed was complete healing of the anastomotic defect with EVT and 90-day mortality. Failure of EVT was defined as persistence of a fistulous orifice requiring an alternative strategy to EVT with drain, or death before confirmation of closure of fistulous orifice. Secondary outcome measures assessed included: time to healing assessed through overall duration of EVT; number of EGDs performed for EVT exchange, percentage of EVT exchanges under GA, complications of EVT (iatrogenic perforation, fistulation, bleeding or inability to tolerate the procedure), duration of admission; mean repositioning interval (days) of EVT drain; feeding route during EVT; postoperative complications were classified according to the Clavien-Dindo (CD) classification, and procedure specific complications assessed [anastomotic stricture, acute respiratory distress syndrome (ARDS), aspiration pneumonia, atrial fibrillation, bleeding, thoracic or abdominal collection, conduit necrosis, chylothorax, hospital acquired pneumonia, recurrent laryngeal nerve palsy]; intensive care unit (ICU) re-admission; hospital re-admission (30 days); re-operation and 30- and 90-day mortality. Return to theatre for thoracic drainage procedures were not considered a failure of EVT provided no anastomotic revision or alternate fistula drainage procedures were performed. AL characteristics were also assessed based on imaging and endoscopic findings including initial diameter defect size of AL, maximal depth of fistulous cavity and distance from dental arch. Statistical analysis was performed using SPSS 29 (IBM, Chicago, IL, USA). Discrete data was expressed as numbers with percentages, with median and interquartile range (IQR) for data with skewed distribution and mean and standard deviation (SD) for normally distributed data. Significance testing for non-parametric continuous data was assessed with Mann-Whitney U test, and Fisher’s exact test was performed for categorical variables. Duration of EVT therapy until treatment success was further assessed by Kaplan-Meier analysis.
EVT insertion technique
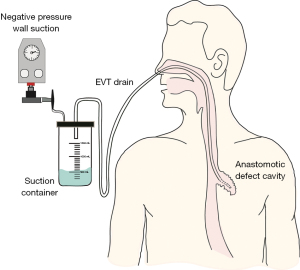
Patients underwent EGD either under GA with endotracheal tube intubation or under sedation with intravenous fentanyl and midazolam. All procedures were performed by a consultant esophagogastric surgeon. The anastomotic defect was directly entered with the scope into the extraluminal cavity when safe entry was possible (Figure 1). The cavity was irrigated with warm water, to reduce bacterial load within the cavity, ensuring the fluid was fully suctioned out. An 18 ch ExudrainÒ (Mediplast, Malmö, Sweden) silicone fenestrated round surgical wound drain was passed trans-nasally to be delivered per orally (Figure 2). A RaptorÒ (US Endoscopy, Mentor, United States) endoscopic grasping forceps was used to hold the tip of the drain and the patient intubated with the gastroscope. The drain was advanced through the anastomotic defect into the cavity under direct vision and secured at the nares with a 2.0 silk suture onto an AMTÒ bridal device (Applied Medical Technology, Ohio, USA). The drain was left in an intraluminal position adjacent to the cavity if the anastomotic defect diameter was too small (<6 mm) to be safely widened to permit passage of the drain (Figure 3). In our practice we do not routinely perform feeding jejunostomy with esophagectomy. Subsequently, to facilitate enteral feeding a nasojejunal FrekaÒ 8 Fr feeding tube (Fresenius Kabi Deutschland GmbH, Hamburg, Germany) may be placed immediately before placing the EVT device. Once placed, the EVT was connected to wall suction at a negative pressure of 200 mmHg [Diamond Suction UnitÒ (Red Back), Therapy Equipment, Norfolk, UK]. Patients are permitted to sip <30 mL of water per hour orally (irrespective of EVT intraluminal or extraluminal placement) and can disconnect from wall suction for one hour daily to mobilise and engage with personal care and physiotherapy. Figure 4 displays an illustrated diagram of the EVT with drain system. A protocol was followed in which the drain was flushed once daily with 20 mL of water to facilitate patency of the drain. Whilst undergoing EVT, patients typically underwent once weekly EGD assessments under sedation or GA. The EVT drain was either left in position or withdrawn by 2 cm and repositioned. If the drain had been initially left in an intraluminal position, but the fistulous orifice was sufficiently wide to accommodate the drain on repeat EGD, the EVT drain was advanced into the cavity. Conversely if the drain is placed initially within the cavity, it may be withdrawn into an intraluminal position abutting the cavity whilst awaiting full closure of the cavity. The drain was withdrawn completely when: the patient was improving with respect to physiological and biochemical status; CT with IV contrast confirmed resolving collection within the cavity and direct visualisation during EGD confirmed the fistulous orifice had epithelialized and would not permit passage of the EVT device (Figure 5). AL features including diameter of fistulous orifice and anatomic location were estimated endoscopically, maximal cavity depth was assessed through CT. The original EVT drain was left in situ for the entire duration of treatment unless it was blocked, in which case it was replaced with a new EVT drain. Reducing exchanges of the EVT drain not only reduces the material cost associated with this treatment strategy but also reduces the procedural time and discomfort for the patient. After completion of EVT, patients were initially monitored on free fluids orally before progression to a soft diet. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethical approval for the collection and reporting of patient healthcare outcomes was obtained under the NorSTRA research database which received a favorable outcome from the Nottingham Research Ethics Committee on 20th August 2020 (20/EM/0193). This permits collection and reporting for research or audit purposes of data collected as part of routine care for patients who have been diagnosed with upper gastrointestinal disease at Norfolk & Norwich University Hospital from 2003 onwards. All patients provided informed written consent for procedures and collection, anonymized storage, and reporting of healthcare outcomes, data, and images.
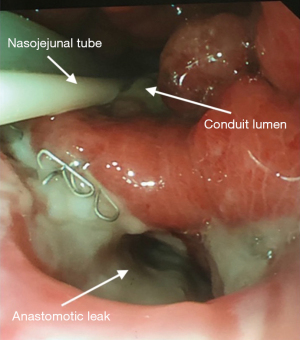
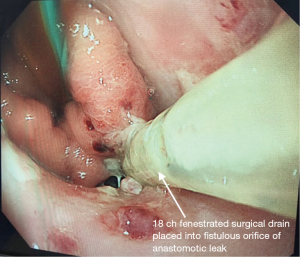
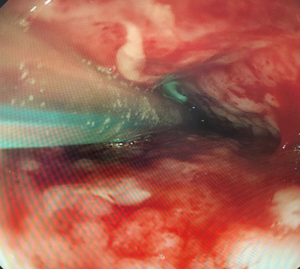
Results
Patient demographics and clinical characteristics
During the study period 29 patients were identified who had experienced an AL following esophagectomy (Figure 6). Of these, five patients had a type III leak and were excluded as they initially underwent thoracotomy for a drainage procedure or for anastomotic diversion. A further three patients with type II leaks were excluded as they were managed solely with endosponge for their AL. Lastly, one patient had a type I leak, which was treated conservatively and was also excluded. Twenty patients remained who had type II leaks managed primarily with EVT using a fenestrated surgical drain. Eighteen male and 2 female patients were included with a median age of 74 years (range, 57–81 years). Nineteen patients had undergone pre-operative chemotherapy with either FLOT-4 (fluorouracil, leucovorin, oxaliplatin and docetaxel) or ECX (epirubicin, cisplatin and capecitabine) regimes before undergoing an Ivor-Lewis esophagectomy for esophageal adenocarcinoma. One patient had no neo-adjuvant chemotherapy or radiotherapy prior to esophagectomy for an esophageal squamous cell carcinoma. Eleven patients underwent minimally invasive esophagectomy with the remaining nine patients undergoing a laparoscopic assisted operation (Table 1).
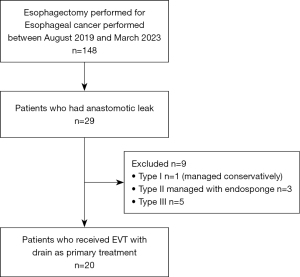
Table 1
| Characteristics | Values (N=20) |
|---|---|
| Age, years, median [range] | 74 [57–81] |
| Sex, n [%] | |
| Male | 18 [90] |
| Female | 2 [10] |
| ASA status, n [%] | |
| Grade II | 2 [10] |
| Grade III | 10 [50] |
| Grade IV | 8 [40] |
| Histology, n [%] | |
| Adenocarcinoma | 19 [95] |
| SCC | 1 [5] |
| Neoadjuvant therapy, n [%] | |
| None | 1 [5] |
| CT | 19 [95] |
| CRT | 0 |
| Esophagectomy, n [%] | |
| Ivor-Lewis | 18 [90] |
| McKeown | 2 [10] |
| Trans-hiatal | 0 |
| Operative approach, n [%] | |
| Minimally Invasive | 9 [45] |
| Laparoscopically assisted | 11 [55] |
| [UICC] cTNM stage for EAC, n [%] | |
| I | 3 [15] |
| II† | 1 [5] |
| IIA | 1 [5] |
| IIB | 1 [5] |
| III | 12 [60] |
| IVA | 2 [10] |
| Clavien-Dindo classification of complications, n [%] | |
| IIIa | 4 [20] |
| IIIb | 12 [60] |
| IV | 4 [20] |
†, (UICC) cTNM stage for SCC. ASA, American Society of Anaesthesiologists; SCC, squamous cell carcinoma; CT, chemotherapy; CRT, chemoradiotherapy; UICC, Union for International Cancer Control; cTNM, clinical tumour node metastasis; EAC, esophageal adenocarcinoma.
EVT and patient outcomes
Outcomes following EVT with drain for the management of type II leaks are presented in Table 2. In this study, a simple fenestrated wound drain inserted into the anastomotic defect cavity and applied to vacuum suction led to full healing of the fistulous orifice in 90% (18/20) of patients. In the two cases where full epithelisation of the fistulous orifice had not occurred patients could not tolerate further EVT procedures. These two patients had undergone EVT therapy for 8 and 17 days respectively. In both instances’ patients had small pin-hole leaks (<6 mm) that had nearly fully epithelialized and subsequently a clinical decision was made to withdraw the EVT drain early for a period of conservative management with nil-per oral diet and observation. In both instances this management was successful, and the patients were safely discharged, without unplanned re-admission.
Table 2
| Characteristics | Values |
|---|---|
| Days between esophagectomy and diagnosis of AL, median [range] | 6.5 [3–20] |
| Days between diagnosis and EVT insertion, median [range] | 1 [0–6] |
| Number of EVT related procedures, median [range] | 3.5 [1–9] |
| Days between EVT repositioning procedures, median [range] | 7 [2–19] |
| Number of EGD investigations during admission, median [range] | 4 [3–8] |
| EVT adjustments performed without GA, n/N [%]† | 34/78 [59] |
| Duration (days) of EVT, median [range] | 19.5 [5–72] |
| Initial intraluminal placement of EVT, n [%] | 7 [35] |
| Initial intracavitary placement of EVT, n [%] | 13 [65] |
| Total number intraluminal placement of EVT, n [%]‡ | 25 [40] |
| Total number intracavitary placement of EVT, n [%]‡ | 38 [60] |
| Defect depth (cm), mean ± SD [range] | 2.26±6.7 [0–9.7] |
| Defect diameter (cm), mean ± SD [range] | 0.91±2.3 [0.5–3.3] |
| 0–0.5 cm, n [%] | 7 [35] |
| 0.6–1 cm, n [%] | 8 [40] |
| 1.1–2 cm, n [%] | 4 [20] |
| >2.1 cm, n [%] | 1 [5] |
| Distance from dental arch (cm), median [range] | |
| Ivor-Lewis esophagectomy | 27 [25–34] |
| McKeown esophagectomy | 22.5 [21–24] |
| Duration (days) of hospital admission, median [range] | 36.5 [15–93] |
| ICU readmission, n [%] | 6 [30] |
| Readmission following discharge (within 30 days), n [%] | 4 [20] |
| In-hospital morality | 0 |
| 90-day mortality | 0 |
| Feeding type, n [%] | |
| Feeding Jejunostomy | 2 [10] |
| Parenteral | 5 [25] |
| Nasojejunal feeding tube | 13 [65] |
| Post-operative complications, n [%] | |
| Anastomotic stricture | 2 [10] |
| ARDS | 2 [10] |
| Aspiration pneumonia | 3 [15] |
| Atrial fibrillation | 9 [45] |
| Bleeding | 0 |
| Collection (intra-thoracic) | 12 [60] |
| Collection (intra-abdominal) | 2 [10] |
| Conduit necrosis | 0 |
| Chylothorax | 1 [5] |
| Hospital acquired pneumonia | 18 [90] |
| Recurrent laryngeal nerve palsy | 0 |
| Complications related to EVT, n [%] | |
| Fistulation(lung/tracheal) | 0 |
| Bleeding | 0 |
| Iatrogenic perforation | 0 |
| Unable to tolerate | 2 [10] |
| Overall successful healing after EVT, n [%] | 18 [90] |
| Additional transthoracic drainage procedures, n [%] | 10 [50] |
| Additional transabdominal drainage procedures, n [%] | 2 [10] |
| Anastomotic revision procedures, n [%] | 0 |
| Adjuvant chemotherapy, n [%] | |
| Completed chemotherapy | 2 [10] |
| Unable to complete chemotherapy | 2 [10] |
| Unfit/declined chemotherapy | 16 [80] |
†, expressed as fraction: number of EGDs with GA/total number of EGDs [%]; ‡, expressed as a fraction of total number of EVT procedures performed. EVT, endoluminal vacuum therapy; AL, anastomotic leak; EGD, esophagogastroduodenoscopy; GA, general anaesthesia; SD, standard deviation; ICU, intensive care unit; ARDS, acute respiratory distress syndrome.
The median time between esophagectomy and diagnosis of AL was 6.5 days (range, 3–20 days). The patient in whom a leak was diagnosed 20 days post-operatively can be considered a significant outlier (Table 2). This patient had a prolonged post-operative admission in ICU with ARDS and initial CT imaging at day 6 and EGD at day 19 post-operatively did not demonstrate AL. The median time difference between diagnosis and insertion of EVT was 1 day (range, 0–6 days), with 75% of patients having this performed within 24 hours of diagnosis. In instances where it was not inserted immediately it was because the defect size was either too small to insert an EVT, which was eventually placed on a subsequent EGD. The median number of EVT related endoscopic procedures was 3.5 (range, 1–9). Patients who had more re-adjustments had slower to heal fistulas and this corresponded with more overall EGDs and longer hospital admissions. The average initial defect size was 0.91 cm (range, 0.5–3.3 cm), all AL’s that had fistulous defects that were <0.6 cm were initially managed with an intraluminal placement of EVT.
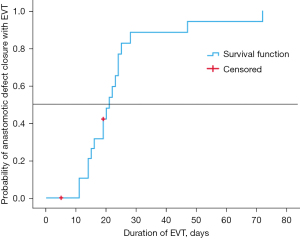
There were a median of 7 days (range, 2–19 days) between EGD for EVT related procedures. The majority (59%) of EGD assessments were performed without GA. The location of EGD and EVT changes varied between the endoscopy department, theatres, and the ICU. Three patients had EVT insertion with X-ray screening guidance drain combined with endoscopy. Typically, if patients were clinically stable and were able to tolerate EGD then the procedure could be performed under sedation. However, in some instances patients were already intubated on ICU or had planned thoracoscopic drainage procedures to be performed in conjunction with EVT changes to optimise GA usage. The median duration of EVT therapy was 19.5 days (range, 5–72 days) with an overall median admission duration of 36.5 days (range, 15–93 days). A Kaplan-Maier analysis of duration of EVT for closure of fistulous orifice after AL is provided in Figure 7. Six patients were re-admitted to the ICU following ward step-down. In all cases, this was for increased monitoring and support following AL diagnosis and for pharmacological management of persistent atrial fibrillation.
Overall, there was 0% mortality amongst this cohort of patients either during hospital admission or at 90-days post-operatively, with all patients having complete healing of the fistula with EVT. Two patients developed stricturing at the level of the anastomosis requiring serial balloon dilatations as outpatients. There were no direct clinical complications associated with EVT such as iatrogenic perforation, bleeding, or the formation of bronchial or tracheal fistulae. Pulmonary complications were common amongst patients in this series given history of AL. Twelve patients developed mediastinal collections with all but two patients developing a hospital acquired pneumonia. These findings were identified through CT imaging obtained during the post-operative period and the severity assessed through clinical examination and analysis of serum biochemistry. For management of these complications, ten patients underwent thoracic drainage procedures post-operatively, the remaining cases were managed with antibiotics alone. In two instances the thoracic drainage procedure consisted of a thoracotomy for intrapleural washout and decortication procedure in one of these cases. Further sub-group analysis comparison of initial intraluminal versus intracavitary placement of EVT is provided in Table 3. Intraluminal placement of EVT drain was typically performed in ALs that had significantly smaller initial defect sizes and defect depths compared to when placed in an intracavitary position. There were no significant differences between initial EVT placement location and EVT duration, complications related to EVT, treatment success or the need for additional thoracic drainage procedures.
Table 3
| Variables | Intraluminal (n=7) | Intracavitary (n=13) | P value |
|---|---|---|---|
| Maximum defect depth (cm), mean (SD) | 1.69 (0.97) | 4.8 (1.89) | <0.001 |
| Initial defect diameter (cm), median (IQR) | 0.5 (0.5) | 0.8 (0.7–1.2) | <0.001 |
| Treatment success, n (%) | 5 (71.4) | 13 (100.0) | 0.11 |
| EVT duration (days), median (IQR) | 19 (16.0–24.0) | 20 (12.5–24.5) | 0.58 |
| Complications related to EVT, n (%) | 2 (28.9) | 0 | 0.11 |
| Additional thoracic drainage procedure performed, n (%) | 4 (57.1) | 6 (46.1) | >0.99 |
EVT, endoluminal vacuum therapy; SD, standard deviation; IQR, interquartile range.
Follow-up
All patient’s electronic health care records were reviewed following discharge to assess for long-term complications for a minimum period of 90-days post-operatively to a maximum of 4 years. Four patients were readmitted following discharge these were for an obstructing food bolus, mild colitis, chylothorax and an intra-abdominal collection requiring US-guided drainage respectively. Two patients developed anastomotic strictures that required balloon dilatation.
Discussion
Key findings
In this series a simple fenestrated surgical wound drain applied to vacuum suction led to full healing of the fistulous tract in 90% of instances of type II AL after esophagectomy. All patients in this series survived to hospital discharge with a 0%, 90-day mortality. There were no serious complications related to EVT with drain use and the median duration of EVT was 19.5 days (range, 5–72 days). The median duration between drain repositioning was 7 days (range, 2–19 days), the majority (56%) of which were performed without GA.
Strengths and limitations
The limitations of this study are analogous to many studies investigating the use of EVT with endosponge (6). Firstly, that as a single centre retrospective study it conveys a lower level of evidence. Secondly, given the small sample size an adequately powered sub-group analysis was not performed. Additionally, as this novel approach was in development during the study period, there may have been a small degree of heterogeneity in the application and delivery of the technique between different surgeons which evolved over time as its clinical effect was appreciated. Although the sample size of the study is small (n=20), it is comparable to other studies that have investigated the use of EVT in single tertiary centres and understandable given that ALs are uncommon complications (6,13). Although limited there is evidence that EVT is a well-tolerated procedure and associated with satisfactory long-term quality of life (17). However, our analysis has not assessed patients experience of undergoing EVT with drain particularly with respect to its impact on restriction of mobility, prolonged periods of nil per oral diet, nasopharyngeal discomfort, and its effect on long-term quality of life. Similar studies investigating EVT use for AL after esophagectomy report an average defect diameter ranging from 1.0–2.54 cm, which is larger than the average defect size of 0.91 cm reported in our investigation (Table 2) (7,18,19). This is likely reflective of an important limitation related to this study in that larger ALs were typically not initially managed with EVT with drain technique within our cohort. Subsequently there is inadequate evidence to demonstrate the effectiveness of the described EVT with drain technique with respect to larger anastomotic defects. Nonetheless this remains the only study describing the use of the EVT with surgical drain approach for AL in the present literature and demonstrates the effectiveness and applicability of this technique.
Comparison with similar research
Meta-analysis of studies investigating EVT with endosponge for leak following esophagectomy report a fistulous closure rate 79.5% (CI: 0.711–0.860) with a range of 60–95% in 14 articles involving 248 patients (6). However, within this cohort of patients there is no standardisation in EVT procedural technique and overall certainty of evidence was rated ‘very low’ as per the GRADE tool. The reported rate of successful fistulous closure in our study is greater than that reported in comparable studies investigating EVT with endosponge. However, given differences in baseline characteristics of patients, leak descriptors such as size of the anastomotic defect, clinical presentation and EVT protocol, a direct comparison between the two techniques would not be appropriate. There is some discrepancy on the definition of fistulous orifice healing between studies. Recent multi-centre prospective registry data for Eso-SpongeÒ adopt a definition that for full healing the defect should have reduced in size to not permit passage of the EVT device and that the surface had epithelialized (7). This seems an appropriate standard to adopt for what constitutes defect healing.
There is debate on appropriate EVT insertion and placement technique. Some authors have recommend widening the anastomotic defect to permit passage of the device to allow for adequate drainage, though other experts advise against extraluminal placement of EVT devices due to the potential risk for associated complications such bronchopulmonary fistula and haemorrhage (20). In this series no such complications were seen despite intracavity placement of the EVT drain in 60% of EGD procedures. In our experience it is difficult to create a standardised approach with respect to the placement of the EVT, which will be dependent on the site of leak, associated size of mediastinal cavity and surgical judgement.
Meta-analysis of studies investigating use of endosponge techniques found a mean duration of stay of 39 days and a mean duration of EVT therapy of 22 days for AL following Esophagectomy (6). Similar durations of EVT treatment of 24.9 days were noted in the Eso-Sponge® registry (7). Our experience has also found similar median durations of therapy using EVT with drain of 19.5 days and duration of admission of 36.5 days respectively. Although the sample size is too small to draw definitive conclusions, the effect of EVT with drain is similar to endosponge approaches with respect to healing rate. An area where the EVT with drain approach may show substantial benefit is by reducing number of EGDs for EVT procedures and overall reduced material cost. Patients had on average four EGDs for drain manipulation with this occurring at a median interval of 7 days (range, 2–19 days). There are some instances where the drain was left in situ without manipulation for prolonged periods of up to 19 days between EGD intervals within this cohort. In these cases, we found drain position could be reliably monitored through CT which avoided the need for frequent repeat EGD. This contrasts with Eso-Sponge® registry data which showed a mean interval of 3.1 days (range, 2–7 days) between sponge exchanges and using on average 7.7 sponges (range, 1–32 sponges) (7). EVT with a simple fenestrated drain as described in our study can be used for the entire duration of treatment and does not mandate drain exchanges. This reduces material cost and the need for frequent repeat EGDs. Although other studies do not explicitly state the proportion of EVT procedures performed under GA, in our series we found that GA was only used in a minority (41%) of instances. This demonstrates that EVT with drain can be performed safely under sedation with experience and appropriate patient selection.
Explanation of findings
Within our unit, management of AL following esophageal resections is now almost exclusively endoscopic with the technique described in this report. Locally this is now the preferred method of management of type II leaks and has diminished the use of SEMS and endosponge devices. A preferable aspect to this approach is it has negated the need to use expensive commercial or custom-made endosponge devices. Unlike endosponge, the drain does not get stuck within the cavity and is relatively simple to insert and remove. Furthermore, the same drain can be left in situ for the entire duration of EVT and does not require change, thereby further reducing the associated material cost and procedural time. Our experience along with the growing popularity of endoscopic management of AL also serves as a reminder of the need for esophagogastric surgeons to develop and maintain advanced endoscopic skills to effectively manage such patients.
Our understanding of this technique has evolved through experience. It was noted that CT scan assessment was able to accurately assess the position of the EVT drain tip within the fistulous cavity and provide an assessment of the size of the cavity and any associated collections, thereby further diminishing the frequency of EGD assessment. Depending on clinical and radiological progress, we would withdraw the position of the drain by approximately 2 cm under endoscopic view thus ensuring that the device was not withdrawn excessively.
We noted a paucity of research on the optimal negative suction pressure proven to optimise healing of the anastomotic defect. In this study we have typically used an initial vacuum pressure of −200 mmHg with wall suction. Similar series using endosponge have utilised lower pressures typically ranging from −75 to −125 mmHg (13,21). Proprietary devices such as Eso-Sponge® also use lower pressures between −100 to −125 mmHg (7). The increased negative vacuum pressure applied in this study, is due to the EVT with drain device being connected to wall suction, which has variable negative pressures, necessitating the use of a higher pressure setting to account for these inconsistencies. Evidence from porcine models suggest that there is no difference in wound diameter after increasing the negative pressure from −75 to −175 mmHg (22). Authors have described the use of commercially available portable negative pressure devices such as Thopaz® (Medela®, Baar, Switzerland) vacuum pump for EVT, which can tolerate pressures of −75 mmHg. This would improve mobility of patients whilst on EVT and may even allow them to leave the ward environment episodically which may improve patient experience and enhance the recovery process (20). Excessive pressures could be postulated to increase the risk of bronchopulmonary fistulation and stricture formation. Additionally, they may cause increased adherence of the sponge material to the surrounding tissues, thereby increasing the force required to extricate the device for exchanges. In this report we note only two instances of stricturing post-operatively requiring dilatation procedures. This may or may not be related to EVT, but nonetheless it would be preferable to use the minimum viable negative pressure to achieve closure of the defect to minimise adverse effects. The higher pressures used in this study may have improved clearance of inflammatory or infectious material and given no sponge was used did not cause issues with adherence of drain to surrounding tissue.
Comparative analysis of EVT with initial placement in Intraluminal versus intracavitary sites demonstrated that EVT was placed in an intraluminal position in instances where there were significantly smaller defects both with respect to defect diameter and cavity depth. Despite this there was no significant differences between initial EVT placement site and other outcome variables including duration of EVT (Table 3). Of note two patients who underwent EVT with intraluminal placement declined further EVT before complete healing of the AL due to discomfort, and although not statistically significant does suggest a need to investigate discomfort symptoms associated with EVT techniques and EVT placement sites in future studies. Additionally, there was no significant difference in leak closure time in those who had EVT initially placed intraluminally (19 days) compared to instances where it was placed intracavitally (20 days) despite significantly smaller defect size in the intraluminal group. This warrants further investigation through adequately powered prospective studies to clarify whether intraluminal placement of EVT has similar efficacy to it use intracavitally.
A frequently overlooked outcome in the management of AL is whether patients were able to recover sufficiently to proceed with adjuvant chemotherapy. Post operative adjuvant chemotherapy improves survival but the associated functional decline with recovery from AL may leave patients unable to pursue this treatment (4). Following oncological evaluation only two patients in this series completed adjuvant chemotherapy, with two patients unable to complete their prescribed course. The remaining sixteen patients were deemed too deconditioned to undergo adjuvant chemotherapy. Further research should consider this important long-term consequence when considering optimal management techniques for managing AL and their potential effect on overall survival.
Implications and actions needed
EVT with drain appears to be a safe and effective technique for managing AL after esophagectomy. However, a standardised reporting framework for EVT procedures and collaborative multi-centre, international, registry data is needed to optimise EVT protocols and clarify indicative parameters for its use. Further research is needed to determine the optimal negative pressure for promoting healing of the fistulous orifice and reducing EVT related complications. Additionally, comparative, and cost-analysis studies with other EVT and other endoscopic strategies are needed to appreciate the overall associated health care costs with management of AL. Further research to identify the optimal management for type II leaks ideally needs to be prospective and utilise a randomised controlled design to perform a direct head-to-head comparison between the various EVT strategies. If such a trial demonstrated non-inferiority, then that may support a wider use of EVT with drain.
Conclusions
EVT with a fenestrated surgical drain achieved full healing in 90% of ALs after esophagectomy with zero mortality in this series. This technique appears to be safe and effective as well as being logistically easier to perform and, in this series, has demonstrated a comparable healing time to endosponge approaches. Negating the use of a sponge-based device, we demonstrate a small number of device changes which can be performed without GA. The benefits of such a technique compared to alternative AL management strategies are numerous through reducing the need for frequent endoscopies and reduced material cost.
Acknowledgments
Data discussed within this manuscript was presented at the European Society Diseases of Esophagus Conference, Leuven February 2023, in poster format entitled ‘No need for a sponge! Endoluminal vacuum therapy using a surgical drain for anastomotic leak after esophagectomy’. The authors would like to acknowledge Suheelan Kulasegaran who assisted with preparation of the poster format (affiliated with Norfolk and Norwich University Hospital).
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://aoe.amegroups.org/article/view/10.21037/aoe-23-24/rc
Data Sharing Statement: https://aoe.amegroups.org/article/view/10.21037/aoe-23-24/dss
Peer Review File: Available at https://aoe.amegroups.com/article/view/10.21037/aoe-23-24/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoe.amegroups.org/article/view/10.21037/aoe-23-24/coif). B.K. serves as an unpaid editorial board member of Annals of Esophagus from December 2023 to November 2025. The others have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethical approval for the collection and reporting of patient healthcare outcomes was obtained under the NorSTRA research database which received a favorable outcome from the Nottingham Research Ethics Committee on 20th August 2020 (20/EM/0193). This permits collection and reporting for research or audit purposes of data collected as part of routine care for patients who have been diagnosed with upper gastrointestinal disease at Norfolk & Norwich University Hospital from 2003 onwards. All patients provided informed written consent for procedures and collection, anonymized storage, and reporting of healthcare outcomes, data, and images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Biere SS, Maas KW, Cuesta MA, et al. Cervical or thoracic anastomosis after esophagectomy for cancer: a systematic review and meta-analysis. Dig Surg 2011;28:29-35. [Crossref] [PubMed]
- Low DE, Kuppusamy MK, Alderson D, et al. Benchmarking Complications Associated with Esophagectomy. Ann Surg 2019;269:291-8. [Crossref] [PubMed]
- Low DE, Alderson D, Cecconello I, et al. International Consensus on Standardization of Data Collection for Complications Associated With Esophagectomy: Esophagectomy Complications Consensus Group (ECCG). Ann Surg 2015;262:286-94. [Crossref] [PubMed]
- Markar S, Gronnier C, Duhamel A, et al. The Impact of Severe Anastomotic Leak on Long-term Survival and Cancer Recurrence After Surgical Resection for Esophageal Malignancy. Ann Surg 2015;262:972-80. [Crossref] [PubMed]
- Watkins JR, Farivar AS. Endoluminal Therapies for Esophageal Perforations and Leaks. Thorac Surg Clin 2018;28:541-54. [Crossref] [PubMed]
- Tavares G, Tustumi F, Tristão LS, et al. Endoscopic vacuum therapy for anastomotic leak in esophagectomy and total gastrectomy: a systematic review and meta-analysis. Dis Esophagus 2021;34:doaa132. [Crossref] [PubMed]
- Richter F, Hendricks A, Schniewind B, et al. Eso-Sponge® for anastomotic leakage after oesophageal resection or perforation: outcomes from a national, prospective multicentre registry. BJS Open 2022;6:zrac030. [Crossref] [PubMed]
- Pournaras DJ, Hardwick RH, Safranek PM, et al. Endoluminal Vacuum Therapy (E-Vac): A Treatment Option in Oesophagogastric Surgery. World J Surg 2018;42:2507-11. [Crossref] [PubMed]
- Pattynama LMD, Eshuis WJ, van Berge Henegouwen MI, et al. Vacuum-stent: A combination of endoscopic vacuum therapy and an intraluminal stent for treatment of esophageal transmural defects. Front Surg 2023;10:1145984. [Crossref] [PubMed]
- Lange J, Dormann A, Bulian DR, et al. VACStent: Combining the benefits of endoscopic vacuum therapy and covered stents for upper gastrointestinal tract leakage. Endosc Int Open 2021;9:E971-6. [Crossref] [PubMed]
- Lange J, Eisenberger CF, Knievel J, et al. Preemptive endoluminal vacuum therapy with the VACStent-A pilot study to reduce anastomotic leakage after Ivor Lewis hybrid esophagectomy. Front Surg 2023;10:1133083. [Crossref] [PubMed]
- Hwang JJ, Jeong YS, Park YS, et al. Comparison of Endoscopic Vacuum Therapy and Endoscopic Stent Implantation With Self-Expandable Metal Stent in Treating Postsurgical Gastroesophageal Leakage. Medicine (Baltimore) 2016;95:e3416. [Crossref] [PubMed]
- Hayami M, Klevebro F, Tsekrekos A, et al. Endoscopic vacuum therapy for anastomotic leak after esophagectomy: a single-center's early experience. Dis Esophagus 2021;34:doaa122. [Crossref] [PubMed]
- Pattynama LMD, Pouw RE, Henegouwen MIVB, et al. Endoscopic vacuum therapy for anastomotic leakage after upper gastrointestinal surgery. Endoscopy 2023;55:1019-25. [Crossref] [PubMed]
- Yin Q, Zhou S, Song Y, et al. Treatment of intrathoracic anastomotic leak after esophagectomy with the sump drainage tube. J Cardiothorac Surg 2021;16:46. [Crossref] [PubMed]
- Shuto K, Kono T, Akutsu Y, et al. Naso-esophageal extraluminal drainage for postoperative anastomotic leak after thoracic esophagectomy for patients with esophageal cancer. Dis Esophagus 2017;30:1-9. [PubMed]
- Dhayat SA, Schacht R, Mennigen R, et al. Long-Term Quality of Life Assessment After Successful Endoscopic Vacuum Therapy of Defects in the Upper Gastrointestinal Tract Quality of Life After EVT. J Gastrointest Surg 2019;23:280-7. [Crossref] [PubMed]
- Min YW, Kim T, Lee H, et al. Endoscopic vacuum therapy for postoperative esophageal leak. BMC Surg 2019;19:37. [Crossref] [PubMed]
- El-Sourani N, Miftode S, Bockhorn M, et al. Endoscopic Management of Anastomotic Leakage after Esophageal Surgery: Ten Year Analysis in a Tertiary University Center. Clin Endosc 2022;55:58-66. [Crossref] [PubMed]
- Gutschow CA, Schlag C, Vetter D. Endoscopic vacuum therapy in the upper gastrointestinal tract: when and how to use it. Langenbecks Arch Surg 2022;407:957-64. [Crossref] [PubMed]
- Jeon JH, Jang HJ, Han JE, et al. Endoscopic Vacuum Therapy in the Management of Postoperative Leakage After Esophagectomy. World J Surg 2020;44:179-85. [Crossref] [PubMed]
- Torbrand C, Ugander M, Engblom H, et al. Wound contraction and macro-deformation during negative pressure therapy of sternotomy wounds. J Cardiothorac Surg 2010;5:75. [Crossref] [PubMed]
Cite this article as: Sivarajan S, Sreedharan L, Kumar B. Endoluminal vacuum therapy using a fenestrated surgical drain for management of anastomotic leak following esophagectomy. Ann Esophagus 2024;7:3.