Assessment of anti-reflux surgery with endoscopy: a narrative review
Introduction
It is estimated that the prevalence of gastro-esophageal reflux disease (GERD) is 10–15% of the Australian population (1,2). Up to 30% of adults experience reflux or reflux-type symptoms, while acknowledging that prevalence is subject to variation in definitions used in studying GERD symptoms (3).
Medical therapy is generally effective in up to 80% of patients, with approximately 20–30% of patients not responding and experiencing persistent symptoms (1). Conservative measures used to address GERD are lifestyle modifications such as weight loss, stress management, and elevation of the head of the bed, with the evidence supporting weight loss as most efficacious treatment (1). Management of confirmed GERD commences with an initial ‘step down’ approach of four to eight weeks of acid suppression treatment [generally proton pump inhibitors (PPIs)], with doses progressively reduced to maintenance level as symptoms respond (3).
Surgical intervention is indicated in patients who have refractory reflux symptoms on maximal medical therapy (such as patients with volume reflux), those who have unwanted side-effects from anti-reflux therapy, or those who want to avoid talking lifelong medication (1,3,4). The use of minimally invasive laparoscopic fundoplication as a surgical intervention for GERD has risen dramatically since its introduction in the early 1990’s (5). In appropriately selected patients, it is highly effective with a 90% satisfaction rate, and a morbidity and mortality rate of less than 0.3% (3,6).
Endoscopy is an essential investigation used in the period following fundoplication if symptoms persist or if new symptoms arise (5,7). Ideally, it is performed by the operating surgeon or an experienced gastroenterologist to assess wrap position and the presence of any hiatal hernia (8). Revisional surgery will be required in 1–5% of the population (9) and an accurate description of the distal esophagus, gastroesophageal junction (GEJ), and fundoplication is critical in determining the cause of failure (5). Endoscopy can reveal twice as many key features as radiography alone, with the objective assessment of post-fundoplication anatomy essential prior to planned revisional surgery (10-13).
However, the description of a fundoplication with endoscopy is not standardized and large variations in reporting are seen (13). A study by Juhasz et al. (5) reported that only 32% of post-surgical endoscopy reports mentioned a previous fundoplication! There is significant deficiency in the reporting of fundoplication anatomy on endoscopy performed by community physicians (5) and a lack of uniformity in the description of endoscopic findings (13). Whilst this may be due to a lack of universally accepted endoscopic terminology, common inadequacies in reporting are also seen, such as ‘fundoplication changes noted’ or ‘hiatus hernia seen’ without any further description or measurements.
This narrative review will examine the current literature for evidence of inter-rater reliability of endoscopic assessment of a prior fundoplication. We will also look for evidence of inter-rater reliability of endoscopy in the assessment of other upper gastrointestinal pathology. This article has been prepared in accordance with the Narrative Review reporting checklist (available at https://aoe.amegroups.com/article/view/10.21037/aoe-22-11/rc).
Methods
Search strategy
Databases searched were Ovid MEDLINE/PubMed, CINAHL, Scopus, Cochrane Database of Systematic Reviews and Web of Science. Development of search terms was aimed to identify papers which focused on the analysis of testing reliability of fundoplication assessment with endoscopy. Inter-rater reliability was defined as the degree of agreement amongst independent observers (in this case, proceduralists performing endoscopy) in the assessment of fundoplication (or other upper gastrointestinal pathologies).
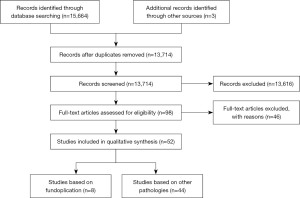
The search terms were developed around four main themes: gastroesophageal reflux disease (GERD), fundoplication, endoscopy, and reliability (Table 1). After removal of duplicates, 13,174 papers were screened by title and abstract. The papers that remained were then reviewed by full text to determine suitability (Figure 1). In addition, comprehensive inclusion and exclusion criteria were determined.
Table 1
| Items | Specification |
|---|---|
| Date of search | (I) 18/02/2021; (II) 23/05/2022 |
| Databases and other sources searched | Ovid MEDLINE/PubMed, CINAHL, Scopus, Cochrane and Web of Science |
| Search terms used | Gastroesophageal Reflux, Esophageal Reflux or GERD or GORD or Gastric Acid Reflux or Gastric Acid Reflux Disease or Gastroesophageal reflux or Gastro esophageal reflux or Gastro esophageal Reflux Disease or Gastro oesophageal reflux or Gastroesophageal Reflux Disease or Reflux, Gastroesophageal |
| Fundoplication or Laparoscopic fundoplication or Open fundoplication or Nissen fundoplication or Total fundoplication or Partial fundoplication | |
| Endoscopy or Upper endoscopy or Endoscopic assessment or Endoscopic evaluation or Endoscopic Surgical Procedure or Endoscopic Surgical Procedures or Endoscopy, Surgical or Surgical Endoscopy or Surgical Procedure, Endoscopic or Surgical Procedures, Endoscopic | |
| Reliab* or Interater reliability or Intrarater reliability or Inter rater reliability or Intra rater reliability or Interobserver reliability or Intraobserver reliability or Inter observer reliability or Intra observer reliability or Inter examiner reliability or Intra examiner reliability or interobserver agreement or validity or assessment or evaluation or agreement | |
| Timeframe | 1990–2022 |
| Inclusion and exclusion criteria |
Inclusion criteria: the papers included in this review were required to discuss interobserver reliability/agreement during endoscopic assessment of ear/nose/throat and upper gastrointestinal pathologies. Fundoplication was the main subject of interest, though other anatomy or pathology assessed on upper gastrointestinal endoscopy were also included. Further inclusion criteria were papers based on primary fundoplication (not revisional fundoplication) and studies based on adult subjects (age >18 years) |
| Exclusion criteria: papers that did not involve use of gastroesophageal endoscopy or did not involve assessment or reliability testing were excluded. Papers that looked at reliability assessment of a questionnaire or tool were reviewed however excluded if there was no component assessing reliability of assessment on endoscopy. One study was excluded as it involved the reclassification of endoscopy reports with a new reporting system, but did not study reliability | |
| Grey literature and unpublished papers were excluded. Papers without access to full text and those not published in English were also excluded. Articles published prior to 1990 were excluded on the premise of seeking up to date evidence in addition to laparoscopic fundoplication not existing prior to this | |
| Selection process | Selection process was conducted by JJ. Fantasia and discussed and reviewed by SK. Thompson |
GERD, gastro-esophageal reflux disease; GORD, gastro-oesophageal reflux disease.
Inclusion criteria
The papers included in this review had to include the assessment of interobserver reliability/agreement during endoscopic assessment of ear/nose/throat and upper gastrointestinal pathologies. Fundoplication was the main subject of interest, although other pathologies were also included. Further inclusion criteria included primary fundoplication (not revisional fundoplication) and adult subjects (age >18 years).
Exclusion criteria
Papers that did not involve the use of endoscopy nor assessment/reliability testing were excluded. One study was excluded as it involved the re-classification of endoscopy reports with a new reporting system but did not study reliability (13). Meeting abstracts and non-English publications were also excluded. Articles published before 1990 were excluded because laparoscopic fundoplication did not exist prior to then.
Results
Fifty-two studies were reviewed, as seen in the PRISMA flow diagram (Figure 1).
Distribution of the studies
Studies varied with regard to the anatomy and/or pathology described, as outlined in Table 2. Eight studies (Table 3) were solely focused on endoscopy related to fundoplication (5,14-20). The most recent study prospectively evaluated inter-rater reliability of fundoplication assessment with endoscopy (18). One study examined the reporting of fundoplication retrospectively (5), while the remaining six studies evaluated the reporting of fundoplication after surgery in a prospective manner, expressed with p-values indicating statistically significant change.
Table 2
| Anatomy/pathology | Studies |
|---|---|
| Fundoplication | Juhasz 2011 (5); Cadière 2009 (14); Csendes 2019 (15); Muls 2013 (16); Petersen 2012 (17); Song 2022 (18); Testoni 2015 (19); Witteman 2013 (20) |
| Barrett’s esophagus | Alvarez Herrero 2009 (21); de Groof 2019 (22); Kato 2017 (23); Lee 2010 (24); Silva 2011 (25); Subramaniam 2020 (26); Trindade 2017 (27); Vahabzadeh 2012 (28) |
| Laryngopharyngeal reflux | Belafsky 2001 (29); Branski 2002 (30); Chang 2015 (31); Kelchner 2007 (32); Lechien 2020 (33); Musser 2011 (34) |
| Esophagitis | Armstrong 1996 (35); Bytzer 1993 (36); Kusano 1999 (37); Lundell 1999 (38); Ma 2022 (39); Pandolfino 2002 (40); Rath 2004 (41); Wasielica-Berger 2018 (42) |
| Secretions/swallowing/oropharyngeal function | Borders 2020 (43); Kaneoka 2013 (44); Leder 2005 (45); Logemann 1999 (46); Miles 2019 (47); Mortensen 2016 (48); Naubauer 2015 (49); Pilz 2016 (50); Pisegna 2018 (51); Starmer 2021 (52); Tohara 2010 (53); Warnecke 2020 (54) |
| Velopharyngeal insufficiency | Miler 2019 (55); Sie 2008 (56); Yoon 2006 (57) |
| Inter arytenoid assessment | Coppess 2019 (58) |
| Laryngopharyngeal sensory discrimination threshold | Cunningham 2007 (59) |
| Esophageal varices | D’Antiga 2015 (60) |
| Radiation induced GI toxicity | Lin 2021 (61) |
| Gastric mucosa atrophy | Miwata 2015 (62) |
| Rhinosinusitis | Parhar 2014 (63) |
| laryngeal, hypopharyngeal, and oropharyngeal lesions | Zwakenberg 2016 (64) |
GI, gastrointestinal.
Table 3
| Author | Raters/endoscopists | Sample size/patients or videos | Classification/grading | Statistical analysis |
|---|---|---|---|---|
| Juhasz 2011 (5) | Not specified | 208 reports | Report review looked forEsophageal caliber and contentsPathologic changesGEJ & relation to fundoplicationEase in traversing the fundoplicationCrural impression if any, symmetryCompetence of fundoplication, slippageGastric contents, evidence of recurrent hiatal hernia | 9 categories of findings Chi-square test (P value) |
| Cadière 2009 (14) | Not specified | 14 patients | Hill grade (GEV)Valve length (apex of the fundus to the valve lip)Wrap circumference | Comparing length and circumferenceMann-Whitney test (P value) |
| Csendes 2019 (15) | Not specified | 150 patients | Esophagitis, cardia type (based on Hill grade) | Fisher’s exact test |
| Muls 2013 (16) | Not specified | 66 patients | Hill grade (GEV)Hiatal hernia gradeEsophagitis grade | Spearman test (P value) |
| Petersen 2012 (17) | 2 | 23 videos | Hill grade (GEV)Wrap circumference (0–360 deg)Quality grade of wrap | Mean scores comparedMann-Whitney U test (P value) |
| Song 2022 (18) | 31 | 20 image sets | Questions based on endoscopic wrap assessment—appearance, position, integrity | Krippendorf alphaCohen’s Kappa |
| Testoni 2015 (19) | Not specified | 50 patients | Hill grade + Jobe length (GEV)Presence of hiatal hernia, esophagitis (LA grading system) | Wilcoxon’s testMann-Whitney testsFisher’s exact test |
| Witteman 2013 (20) | Not specified | 15 patients | Hiatal herniaEsophagitis (LA grading scale)Appearance of the fundoplication | Simply presented as categorical counts |
GEJ, gastroesophageal junction; GEV, gastroesophageal valve; LA, Los Angeles classification.
Of the remaining 44 studies (Table 4) included in the broader search of the literature, 8 were on Barrett’s esophagus (21-28), 6 were on laryngopharyngeal reflux (29-34), 8 were on esophagitis (35-42), 12 were on secretions or swallowing/oropharyngeal function (43-54), 3 on velopharyngeal insufficiency (55-57) and the remainder on various ear/nose/throat and upper gastrointestinal pathologies (58-64).
Table 4
| Anatomy/pathology | Author | Raters/endoscopists | Sample size/patients or videos | Classification/grading | Inter-observer | Intra-observer |
|---|---|---|---|---|---|---|
| Barrett’s esophagus | Alvarez Herrero 2009 (21) | 8 | 200 images | Simplified classification (regularity of mucosal and vascular patterns) | Kappa | Kappa |
| de Groof 2019 (22) | 6 | 40 images | VAS for macroscopic appearance, surface relief, lesions, delineations | Paired t-test, Wilcoxon signed rank test, McNemar test, Spearman’s rank | ||
| Kato 2017 (23) | 4 | 248 images | NBI classification (narrow band imaging) | Kappa | Kappa | |
| Lee 2010 (24) | 34 | 21 videos | Prague C & M Criteria; location of GEJ and diaphragmatic hiatus | ICC | – | |
| Silva 2011 (25) | 9 | 84 videos | Grading system (Kanzas, Amsterdam or Nottingham); Prediction—Histological, certainty, time | Cohen’s kappa, ICC | – | |
| Subramaniam 2020 (26) | 10 | 50 images | BLINC | Kappa | Kappa | |
| Trindade 2017 (27) | 8 | 120 images | Mucosal assessment | Kappa | ||
| Vahabzadeh 2012 (28) | 18 | 18 videos | Prague C & M Criteria; location of GEJ and diaphragmatic hiatus | ICC | – | |
| Laryngo-pharyngeal reflux | Belafsky 2001 (29) | 2 | 40 patients | 8 items—RFS | Pearson product moment | Pearson product moment |
| Branski 2002 (30) | 5 | 120 videos | degree of erythema and degree of edema for inter-arytenoid pachydermia; likelihood/severity of LPRD | ICC | Kendall bivariate | |
| Chang 2015 (31) | 10 | 30 videos | 8 items—RFS | Leiss’s ICC, Multi rater kappa | – | |
| Kelchner 2007 (32) | 4 | 30 videos | 8 items—RFS | ICC, McNemar’s statistic, Log linear regression | – | |
| Lechien 2020 (33) | 5 | 106 videos | 3 parts—RSA | Kendall’s Concordance | Spearman’s rank | |
| Musser 2011 (34) | 3 | 36 videos | 8 items—RFS | Cohen’s kappa, ICC | Cohen’s kappa, ICC | |
| Esophagitis | Armstrong 1996 (35) | 59 | 123 images/videos | LA classification | Cohen kappa | |
| Bytzer 1993 (36) | 3 | 150 patients | Savary-Miller classification | Kappa | ||
| Kusano 1999 (37) | 21 | 50 images | LA classification | Kappa | ||
| Lundell 1999 (38) | 46 | 22 videos | Endoscopic classification | Kappa | – | |
| Ma 2022 (39) | 2 | 42 videos | EREFS | ICC | ICC | |
| Pandolfino 2002 (40) | 9 | 235 images | LA and Hetzel-dent classification | Kappa | Kappa | |
| Rath 2004 (41) | 9 | 60 patients | LA and Savary-Miller classification; MUSE scoring systems | Kappa | ||
| Wasielica-Berger 2018 (42) | 4 | 56 images | LA classification | Kappa | ||
| Secretions/swallowing/oropharyngeal function | Borders 2020 (43) | 4 | 125 videos | Presence, absence or inability to rate LAR | Fleiss’ kappa, Cohen’s kappa | ICC Cohen’s kappa |
| Kaneoka 2013 (44) | 4 | 63 patients | Boston Residue and Clearance Scale during FEES | ICC | ICC | |
| Leder 2005 (45) | 3 | 20 patients | FEES | Kappa | ||
| Logemann 1999 (46) | 2 | 3 patients | FEES | Paired t-test | ||
| Miles 2019 (47) | 28 | 10 videos | Secretion Scale | ICC | ICC | |
| Mortensen 2016 (48) | 2 | 33 patients | Swallowing Assessment of Saliva Scale | Kappa | ||
| Naubauer 2015 (49) | 20 | 261 | Yale Pharyngeal Residue Severity Rating Scale during FEES | Kappa | Kappa | |
| Pilz 2016 (50) | 2 | 60 videos | Four ordinal FEES variables | Linear weighted kappa coefficient | Linear weighted kappa coefficient | |
| Pisegna 2018 (51) | 44 | 81 videos | FEES | ICC | Kappa | |
| Starmer 2021 (52) | 3 | 100 patients | Dynamic imaging grade | Quadratic weighted kappa | Quadratic weighted kappa | |
| Tohara 2010 (53) | 9 | 10 patients | 16 points—FEES | Cohen’s kappa | Cohen’s kappa | |
| Warnecke 2020 (54) | 33 | 10 videos | Categorical variables | Krippendorff alpha | Light’s K | |
| Velopharyngeal insufficiency | Miler 2019 (55) | 16 | 50 videos | Golding Kushner Scale | ICC, Fleiss’ kappa | |
| Sie 2008 (56) | 16 | 50 videos | Golding Kushner Scale | ICC, kappa coefficient | ICC | |
| Yoon 2006 (57) | 6 | 50 videos | Golding Kushner Scale | ICC, kappa coefficient | ICC | |
| Rhinosinusitis | Parhar 2014 (63) | 5 | 50 images | P-J staging | ICC | Fleiss’ kappa |
| Inter arytenoid assessment | Coppess 2019 (58) | 4 | 30 videos | Interarytenoid assessment protocol | Cohen kappa | Cohen kappa |
| Laryngo-pharyngeal sensory discrimination threshold | Cunningham 2007 (59) | 3 | 27 patients | LPSDT | Spearman Rank | Spearman Rank |
| Esophageal varices | D’Antiga 2015 (60) | 10 | 100 images | Classification A and B Scales | Fleiss’ Kappa, Cohen Kappa | |
| Radiation induced GI toxicity | Lin 2021 (61) | 2 | 19 patients | Toxicity scoring system | Kappa, Gwet’s AC1 | |
| Gastric mucosa atrophy | Miwata 2015 (62) | 12 | 91 patients | Kimura-Takemoto Classification | Kappa | Kappa |
| Laryngeal, oro/hypo pharyngeal lesions | Zwakenberg 2016 (64) | 12 | 100 images | Lesion assessment | Fleiss’ Kappa | Cohen Kappa |
VAS, Visual Analog Scale; GEJ, gastroesophageal junction; GI, gastrointestinal; NBI, narrow band imaging; GEJ, gastroesophageal junction; BLINC, Blue Light Imaging for Barrett’s Neoplasia Classification; RFS, Reflux Finding Score; LPRD, laryngo-pharyngeal reflux disease; RSA, Reflux Sign Assessment; LA, Los Angeles classification; EREFS, Endoscopic Reference Score: edema, rings, exudate, furrows, stricture; MUSE, metaplasia, ulcer, stricture, erosion; LAR, laryngeal-adductor reflex; FEES, fiberoptic endoscopic evaluation of swallowing; LPSDT, laryngopharyngeal sensory discrimination threshold test; ICC, intraclass correlation coefficient.
Fundoplication study findings
Song et al. 2022 (18) evaluated the accuracy of the endoscopic assessment of Nissen fundoplication integrity. Thirty-one participants (gastroenterology fellows, subspecialists and foregut surgeons) scored fundoplication anatomy from 20 image sets. They found that diagnostic confidence was considerably varied with poor inter-rater agreement (low to no agreement), and a Krippendorf’s alpha less than 0.3. Intra-rater reliability between paired images varied from none to moderate agreement (kappa range, 0 to 0.67).
The study by Juhasz et al. was a retrospective review study. It involved the assessment of 208 endoscopy reports, performed by general endoscopists outside the specialist center and by upper gastrointestinal surgeons from within the specialist center (5). The authors found inadequate reporting of fundoplication by general endoscopists, with an alarming 68% failing to report the presence of a fundoplication (P<0.05).
The study by Petersen et al. was designed to evaluate the fundoplication constructed following an experimental transoral endoscopic procedure using the EsophyXTM device (17). The study involved two independent investigators reporting on intraoperative videos of 23 patients, on two occasions (pre- and post-transoral fundoplication). Assessment of the fundoplication involved three features: Hill grade [defined as Type I—prominent fold of tissue along the lesser curvature next to the endoscope; Type II—fold is less prominent and there are periods of opening and rapid closing around the endoscope; Type III—fold is not prominent and the endoscope is not tightly gripped by the tissue; Type IV—no fold, and the lumen of the esophagus is open, hiatal hernia is always present (65)], estimated measure of circumference of the wrap, and overall quality grade of the wrap. The authors did not look at inter-rater reliability.
Csendes et al. evaluated the objective appearance of a Nissen fundoplication fifteen years postoperatively with endoscopy (15), while remaining four studies evaluated the fundoplication constructed endoscopically (EsophyXTM) (14,16,19,20). None of studies disclosed how many raters assessed the fundoplication. Based on the design methods, the assessments were made by the endoscopist performing the procedure, rather than by video review, as was the case in the study by Petersen et al. (17). All of the remaining studies (14,16,19,20) reported similar results supporting feasibility of the procedure.
It is important to note that the recent study by Song et al. is the only study with a robust design to evaluate inter-rater reliability of fundoplication assessment with endoscopy (18). The authors of the remaining seven studies presented comparative statistical analysis in the form of P values, which are representative of a statistical comparison and association rather than specific inter-rater reliability.
Non-fundoplication study findings
The 44 remaining studies, whilst not assessing fundoplication per se, were designed to determine the inter-rater and/or intra-rater reliability in the assessment and reporting of other upper gastrointestinal pathologies on endoscopy (Table 4). The studies all had a similar design methodology, although they each describe different assessment tools, leading to the use of different statistical analyses of reliability.
The correlation coefficients used to determine reliability were; Kappa, Krippendorff alpha, Light’s kappa, Cohen’s kappa, Intra-class correlation coefficient, Fleiss’ kappa, Kendal’s W and Spearman Rank correlation coefficient.
Discussion
The aim of this narrative review was to examine the current literature for evidence of inter-rater reliability in the assessment of fundoplication assessment with endoscopy. Our secondary aims were to look for any literature on reliability measures when assessing upper gastrointestinal pathology (i.e., not necessarily fundoplication).
We confirmed our suspicion that there is a paucity of data on the reliability of fundoplication assessment with endoscopy. Juhasz et al. (5) made the same observation in their study, finding a low percentage of general endoscopists routinely identified the presence of a fundoplication in their endoscopy report. They recommended a standardized proforma to assess not only the presence of a fundoplication, but its integrity and anatomical features in their more recent paper (13). To our knowledge, however, this assessment tool has not been validated externally.
The methodological design varied considerably across the 52 studies included in our narrative review. The initial eight studies which examined fundoplication included a small number of raters, which is associated with low statistical power and reduced veracity of findings (66). These studies were not powered to evaluate reliability between raters, rather they were designed to compare the appearance of the fundoplication pre- and post-intervention. In contrast, the subsequent forty-four studies were designed to examine the reliability between raters when assessing upper gastrointestinal pathology. These studies therefore had a higher number of raters to allow for a more powerful calculation of reliability, and all of these studies used statistical coefficients to determine inter-rater reliability.
Statistical coefficients are a critical component of reliability studies because they determine the level of agreement between different evaluations from a response variable (67). Whilst this may seem similar to comparing results on two occasions (with P values), the important distinction between definitions is the ‘level of agreement’. This agreement is important in providing evidence of the closeness of results, rather than simply an expression of results. Established correlation coefficients therefore demonstrate reliability, where higher values demonstrate greater reliability and a smaller error of between subject variability (68).
Conclusions
This narrative review has identified a paucity of literature on the reliability of endoscopic assessment of laparoscopic fundoplication. Of the eight studies which looked at fundoplication, one was designed to assess reliability (albeit with a low number of assessors), and seven had their design focused on comparison pre-and post-operatively rather than the specific reliability of assessors. The remaining 44 studies in our review confirmed that reliability studies are feasible when using endoscopy to assess other gastrointestinal pathologies. These studies will help provide a framework for the development of inter-rater reliability studies in the assessment of fundoplication with endoscopy.
It is important that general endoscopists achieve a high standard when reporting the presence and appearance of a fundoplication. These reports form part of the patient’s medical record and are essential in the patient’s individualized care. In addition to documentation of the location of the squamocolumnar junction, and findings in the lower esophagus (e.g., ulcerative esophagitis/Barrett’s esophagus), the endoscopist should describe the appearance of the fundoplication (i.e., intact, partially intact, or disrupted), the position of the wrap (i.e., above, at, or below the level of the diaphragm), and the presence or not of a hiatus hernia (in the retroflexed position). Images should also be taken to support the description and to serve as a baseline for future comparison. It is equally important that a robust classification system is developed and/or validated for universal reporting.
Acknowledgments
The authors thank Dr. Aliese Millington for her assistance in the development of the database search strategy.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://aoe.amegroups.com/article/view/10.21037/aoe-22-11/rc
Peer Review File: Available at https://aoe.amegroups.com/article/view/10.21037/aoe-22-11/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://aoe.amegroups.com/article/view/10.21037/aoe-22-11/coif). S.K.T. serves as an unpaid editorial board member of Annals of Esophagus from September 2019 to August 2025. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Keung C, Hebbard G. The management of gastro-oesophageal reflux disease. Aust Prescr 2016;39:6-10. [Crossref] [PubMed]
- Miller G, Wong C, Pollack A. Gastro-oesophageal reflux disease (GORD) in Australian general practice patients. Aust Fam Physician 2015;44:701-4. [PubMed]
- Piterman L, Nelson M, Dent J. Gastro-oesophageal reflux disease--current concepts in management. Aust Fam Physician 2004;33:987-91. [PubMed]
- Fisichella PM, Patti MG. GERD procedures: when and what? J Gastrointest Surg 2014;18:2047-53. [Crossref] [PubMed]
- Juhasz A, Sundaram A, Hoshino M, et al. Endoscopic assessment of failed fundoplication: a case for standardization. Surg Endosc 2011;25:3761-6. [Crossref] [PubMed]
- Dent J, Brun J, Fendrick A, et al. An evidence-based appraisal of reflux disease management--the Genval Workshop Report. Gut 1999;44:S1-16. [Crossref] [PubMed]
- Galvani C, Fisichella PM, Gorodner MV, et al. Symptoms are a poor indicator of reflux status after fundoplication for gastroesophageal reflux disease: role of esophageal functions tests. Arch Surg 2003;138:514-8; discussion 518-9. [Crossref] [PubMed]
- Kaiser LR, Jamieson G, Thompson SK. Operative Thoracic Surgery. 6 ed. Boca Raton: Boca Raton: CRC Press; 2018.
- Furnée EJ, Draaisma WA, Broeders IA, et al. Surgical reintervention after failed antireflux surgery: a systematic review of the literature. J Gastrointest Surg 2009;13:1539-49. [Crossref] [PubMed]
- Iqbal A, Awad Z, Simkins J, et al. Repair of 104 failed anti-reflux operations. Ann Surg 2006;244:42-51. [Crossref] [PubMed]
- Jailwala J, Massey B, Staff D, et al. Post-fundoplication symptoms: the role for endoscopic assessment of fundoplication integrity. Gastrointest Endosc 2001;54:351-6. [Crossref] [PubMed]
- Jobe BA, Kahrilas PJ, Vernon AH, et al. Endoscopic appraisal of the gastroesophageal valve after antireflux surgery. Am J Gastroenterol 2004;99:233-43. [Crossref] [PubMed]
- Mittal SK, Juhasz A, Ramanan B, et al. A proposed classification for uniform endoscopic description of surgical fundoplication. Surg Endosc 2014;28:1103-9. [Crossref] [PubMed]
- Cadière GB, Van Sante N, Graves JE, et al. Two-year results of a feasibility study on antireflux transoral incisionless fundoplication using EsophyX. Surg Endosc 2009;23:957-64. [Crossref] [PubMed]
- Csendes A, Orellana O, Cuneo N, et al. Long-term (15-year) objective evaluation of 150 patients after laparoscopic Nissen fundoplication. Surgery 2019;166:886-94. [Crossref] [PubMed]
- Muls V, Eckardt AJ, Marchese M, et al. Three-year results of a multicenter prospective study of transoral incisionless fundoplication. Surg Innov 2013;20:321-30. [Crossref] [PubMed]
- Petersen RP, Filippa L, Wassenaar EB, et al. Comprehensive evaluation of endoscopic fundoplication using the EsophyX™ device. Surg Endosc 2012;26:1021-7. [Crossref] [PubMed]
- Song EJ, Yadlapati R, Chen JW, et al. Variability in endoscopic assessment of Nissen fundoplication wrap integrity and hiatus herniation. Dis Esophagus 2022;35:doab078. [Crossref] [PubMed]
- Testoni PA, Testoni S, Mazzoleni G, et al. Long-term efficacy of transoral incisionless fundoplication with Esophyx (Tif 2.0) and factors affecting outcomes in GERD patients followed for up to 6 years: a prospective single-center study. Surg Endosc 2015;29:2770-80. [Crossref] [PubMed]
- Witteman BP, Kessing BF, Snijders G, et al. Revisional laparoscopic antireflux surgery after unsuccessful endoscopic fundoplication. Surg Endosc 2013;27:2231-6. [Crossref] [PubMed]
- Alvarez Herrero L, Curvers WL, Bansal A, et al. Zooming in on Barrett oesophagus using narrow-band imaging: an international observer agreement study. Eur J Gastroenterol Hepatol 2009;21:1068-75. [Crossref] [PubMed]
- de Groof AJ, Swager AF, Pouw RE, et al. Blue-light imaging has an additional value to white-light endoscopy in visualization of early Barrett's neoplasia: an international multicenter cohort study. Gastrointest Endosc 2019;89:749-58. [Crossref] [PubMed]
- Kato M, Goda K, Shimizu Y, et al. Image assessment of Barrett's esophagus using the simplified narrow band imaging classification. J Gastroenterol 2017;52:466-75. [Crossref] [PubMed]
- Lee CK, Chung IK, Lee SH, et al. Endoscopic partial resection with the unroofing technique for reliable tissue diagnosis of upper GI subepithelial tumors originating from the muscularis propria on EUS (with video). Gastrointest Endosc 2010;71:188-94. [Crossref] [PubMed]
- Silva FB, Dinis-Ribeiro M, Vieth M, et al. Endoscopic assessment and grading of Barrett's esophagus using magnification endoscopy and narrow-band imaging: accuracy and interobserver agreement of different classification systems (with videos). Gastrointest Endosc 2011;73:7-14. [Crossref] [PubMed]
- Subramaniam S, Kandiah K, Schoon E, et al. Development and validation of the international Blue Light Imaging for Barrett's Neoplasia Classification. Gastrointest Endosc 2020;91:310-20. [Crossref] [PubMed]
- Trindade AJ, Inamdar S, Smith MS, et al. Volumetric laser endomicroscopy in Barrett's esophagus: interobserver agreement for interpretation of Barrett's esophagus and associated neoplasia among high-frequency users. Gastrointest Endosc 2017;86:133-9. [Crossref] [PubMed]
- Vahabzadeh B, Seetharam AB, Cook MB, et al. Validation of the Prague C & M criteria for the endoscopic grading of Barrett's esophagus by gastroenterology trainees: a multicenter study. Gastrointest Endosc 2012;75:236-41. [Crossref] [PubMed]
- Belafsky PC, Postma GN, Koufman JA. The validity and reliability of the reflux finding score (RFS). Laryngoscope 2001;111:1313-7. [Crossref] [PubMed]
- Branski RC, Bhattacharyya N, Shapiro J. The reliability of the assessment of endoscopic laryngeal findings associated with laryngopharyngeal reflux disease. Laryngoscope 2002;112:1019-24. [Crossref] [PubMed]
- Chang BA, MacNeil SD, Morrison MD, et al. The Reliability of the Reflux Finding Score Among General Otolaryngologists. J Voice 2015;29:572-7. [Crossref] [PubMed]
- Kelchner LN, Horne J, Lee L, et al. Reliability of speech-language pathologist and otolaryngologist ratings of laryngeal signs of reflux in an asymptomatic population using the reflux finding score. J Voice 2007;21:92-100. [Crossref] [PubMed]
- Lechien JR, Rodriguez Ruiz A, Dequanter D, et al. Validity and Reliability of the Reflux Sign Assessment. Ann Otol Rhinol Laryngol 2020;129:313-25. [Crossref] [PubMed]
- Musser J, Kelchner L, Neils-Strunjas J, et al. A comparison of rating scales used in the diagnosis of extraesophageal reflux. J Voice 2011;25:293-300. [Crossref] [PubMed]
- Armstrong D, Bennett JR, Blum AL, et al. The endoscopic assessment of esophagitis: a progress report on observer agreement. Gastroenterology 1996;111:85-92. [Crossref] [PubMed]
- Bytzer P, Havelund T, Hansen JM. Interobserver variation in the endoscopic diagnosis of reflux esophagitis. Scand J Gastroenterol 1993;28:119-25. [Crossref] [PubMed]
- Kusano M, Ino K, Yamada T, et al. Interobserver and intraobserver variation in endoscopic assessment of GERD using the "Los Angeles" classification. Gastrointest Endosc 1999;49:700-4. [Crossref] [PubMed]
- Lundell LR, Dent J, Bennett JR, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut 1999;45:172-80. [Crossref] [PubMed]
- Ma C, Bredenoord AJ, Dellon ES, et al. Reliability and responsiveness of endoscopic disease activity assessment in eosinophilic esophagitis. Gastrointest Endosc 2022;95:1126-1137.e2. [Crossref] [PubMed]
- Pandolfino JE, Vakil NB, Kahrilas PJ. Comparison of inter- and intraobserver consistency for grading of esophagitis by expert and trainee endoscopists. Gastrointest Endosc 2002;56:639-43. [Crossref] [PubMed]
- Rath HC, Timmer A, Kunkel C, et al. Comparison of interobserver agreement for different scoring systems for reflux esophagitis: Impact of level of experience. Gastrointest Endosc 2004;60:44-9. [Crossref] [PubMed]
- Wasielica-Berger J, Kemona A, Kiśluk J, et al. The added value of magnifying endoscopy in diagnosing patients with certain gastroesophageal reflux disease. Adv Med Sci 2018;63:359-66. [Crossref] [PubMed]
- Borders JC, O'Dea MB, McNally E, et al. Inter- and Intra-Rater Reliability of Laryngeal Sensation Testing with the Touch Method During Flexible Endoscopic Evaluations of Swallowing. Ann Otol Rhinol Laryngol 2020;129:565-71. [Crossref] [PubMed]
- Kaneoka AS, Langmore SE, Krisciunas GP, et al. The Boston Residue and Clearance Scale: preliminary reliability and validity testing. Folia Phoniatr Logop 2013;65:312-7. [Crossref] [PubMed]
- Leder SB, Acton LM, Lisitano HL, et al. Fiberoptic endoscopic evaluation of swallowing (FEES) with and without blue-dyed food. Dysphagia 2005;20:157-62. [Crossref] [PubMed]
- Logemann JA, Rademaker AW, Pauloski BR, et al. Interobserver agreement on normal swallowing physiology as viewed by videoendoscopy. Folia Phoniatr Logop 1999;51:91-8. [Crossref] [PubMed]
- Miles A, Hunting A. Development, intra- and inter-rater reliability of the New Zealand Secretion Scale (NZSS). Int J Speech Lang Pathol 2019;21:377-84. [Crossref] [PubMed]
- Mortensen J, Jensen D, Kjaersgaard A. A validation study of the Facial-Oral Tract Therapy Swallowing Assessment of Saliva. Clin Rehabil 2016;30:410-5. [Crossref] [PubMed]
- Neubauer PD, Rademaker AW, Leder SB. The Yale Pharyngeal Residue Severity Rating Scale: An Anatomically Defined and Image-Based Tool. Dysphagia 2015;30:521-8. [Crossref] [PubMed]
- Pilz W, Vanbelle S, Kremer B, et al. Observers' Agreement on Measurements in Fiberoptic Endoscopic Evaluation of Swallowing. Dysphagia 2016;31:180-7. [Crossref] [PubMed]
- Pisegna JM, Borders JC, Kaneoka A, et al. Reliability of Untrained and Experienced Raters on FEES: Rating Overall Residue is a Simple Task. Dysphagia 2018;33:645-54. [Crossref] [PubMed]
- Starmer HM, Arrese L, Langmore S, et al. Adaptation and Validation of the Dynamic Imaging Grade of Swallowing Toxicity for Flexible Endoscopic Evaluation of Swallowing: DIGEST-FEES. J Speech Lang Hear Res 2021;64:1802-10. [Crossref] [PubMed]
- Tohara H, Nakane A, Murata S, et al. Inter- and intra-rater reliability in fibroptic endoscopic evaluation of swallowing. J Oral Rehabil 2010;37:884-91. [Crossref] [PubMed]
- Warnecke T, Muhle P, Claus I, et al. Inter-rater and test-retest reliability of the "standardized endoscopic swallowing evaluation for tracheostomy decannulation in critically ill neurologic patients". Neurol Res Pract 2020;2:9. [Crossref] [PubMed]
- Miller C, Bly R, Cofer S, et al. Multicenter Interrater Reliability in the Endoscopic Assessment of Velopharyngeal Function Using a Video Instruction Tool. Otolaryngol Head Neck Surg 2019;160:720-8. [Crossref] [PubMed]
- Sie KC, Starr JR, Bloom DC, et al. Multicenter interrater and intrarater reliability in the endoscopic evaluation of velopharyngeal insufficiency. Arch Otolaryngol Head Neck Surg 2008;134:757-63. [Crossref] [PubMed]
- Yoon PJ, Starr JR, Perkins JA, et al. Interrater and intrarater reliability in the evaluation of velopharyngeal insufficiency within a single institution. Arch Otolaryngol Head Neck Surg 2006;132:947-51. [Crossref] [PubMed]
- Coppess S, Padia R, Horn D, et al. Standardizing Laryngeal Cleft Evaluations: Reliability of the Interarytenoid Assessment Protocol. Otolaryngol Head Neck Surg 2019;160:533-9. [Crossref] [PubMed]
- Cunningham JJ, Halum SL, Butler SG, et al. Intraobserver and interobserver reliability in laryngopharyngeal sensory discrimination thresholds: a pilot study. Ann Otol Rhinol Laryngol 2007;116:582-8. [Crossref] [PubMed]
- D'Antiga L, Betalli P, De Angelis P, et al. Interobserver Agreement on Endoscopic Classification of Oesophageal Varices in Children. J Pediatr Gastroenterol Nutr 2015;61:176-81. [Crossref] [PubMed]
- Lin D, Moningi S, Abi Jaoude J, et al. Development of an Objective Scoring System for Endoscopic Assessment of Radiation-Induced Upper Gastrointestinal Toxicity. Cancers (Basel) 2021;13:2136. [Crossref] [PubMed]
- Miwata T, Quach DT, Hiyama T, et al. Interobserver and intraobserver agreement for gastric mucosa atrophy. BMC Gastroenterol 2015;15:95. [Crossref] [PubMed]
- Parhar HS, Thamboo A, Habib AR, et al. The interrater and intrarater reliability of the Philpott-Javer staging system based on level of training. Otolaryngol Head Neck Surg 2014;150:538-41. [Crossref] [PubMed]
- Zwakenberg MA, Dikkers FG, Wedman J, et al. Narrow band imaging improves observer reliability in evaluation of upper aerodigestive tract lesions. Laryngoscope 2016;126:2276-81. [Crossref] [PubMed]
- Hansdotter I, Björ O, Andreasson A, et al. Hill classification is superior to the axial length of a hiatal hernia for assessment of the mechanical anti-reflux barrier at the gastroesophageal junction. Endosc Int Open 2016;4:E311-7. [Crossref] [PubMed]
- Button KS, Ioannidis JP, Mokrysz C, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci 2013;14:365-76. [Crossref] [PubMed]
- Bujang MA, Baharum N. Guidelines of the minimum sample size requirements for Kappa agreement test. Epidemiology, biostatistics, and public health 2017;14: [Crossref]
- Walter SD, Eliasziw M, Donner A. Sample size and optimal designs for reliability studies. Stat Med 1998;17:101-10. [Crossref] [PubMed]
Cite this article as: Fantasia JJ, Thompson SK. Assessment of anti-reflux surgery with endoscopy: a narrative review. Ann Esophagus 2023;6:43.