Endoscopic palliation of esophageal cancer: a narrative review apart from stenting
Introduction
Esophageal cancer remains the eighth-most common cancer and the sixth-most common cause of death worldwide. Prevalence within the United States alone is estimated to be 19,260 cases and expected to result in 15,530 deaths during 2021 (1). While surgery and neoadjuvant chemotherapy and radiation remain an important cornerstone in the treatment algorithm for many patients with early stage and locoregional disease, most patients are diagnosed at advanced stages, and non-surgical palliative treatment modalities become an important part of the physicians’ armamentarium. Dysphagia and bleeding resulting in hematemesis and anemia can significantly impact quality of life in these patients. Endoluminal treatment options have become increasingly important, as these modalities allow dysphagia, risk of aspiration, and resulting malnutrition to be resolved to varying degrees. In this paper, we will focus on endoluminal ablative options such as photodynamic therapy (PDT) and cryotherapy. Esophageal stenting will be discussed elsewhere in this series. We present the following article in accordance with the Narrative Review reporting checklist (available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-51/rc).
PDT
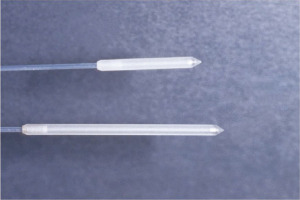
PDT is a minimally invasive form of treatment used for many non-oncologic and oncologic diseases. In the esophagus, this can be used with curative intent for patients with high-grade dysplasia and for patients with superficial tumors who are not candidates for resection (2). In addition, and of relevance to this topic, PDT can be very helpful in palliation of esophageal cancer. PDT is a multistep process. First, a photosensitizer is administered to patients intravenously. Currently the only FDA approved photosensitizer for esophageal use is porfimer sodium. The photosensitizer selectively accumulates within tumor cells and at optimal concentrations within 48 hours of administration. After 24–48 hours of administering the photosensitizer, flexible endoscopy is performed and light therapy is delivered endoluminally using a cylindrical diffuser light fiber (Figure 1). Light at a specific wavelength of 630 nanometers penetrates the tissue containing porfimer sodium and induces a cascade of chemical reactions that results in formation of highly reactive oxygen species, which destroys cellular structures resulting in PDT’s tumoricidal effects. A repeat endoscopy is performed 48 hours later. Usually necrotic tissue is observed in the esophageal lumen in response to the light therapy. The necrotic tissue is debrided and irrigated and an additional light therapy treatment can be delivered to any viable tumor that is present. In many cases, a 3rd endoscopy repeating this process is performed another 48 hours later. This process: injection, endoscopy/PDT treatment, endoscopy/debridement/additional PDT treatment is referred to as a treatment course. In some instances, patients may require additional treatment courses (repeat injection and endoscopic treatments) (3). The ideal candidate for PDT will be a patient who has primarily endoluminal disease. PDT is less effective for tumors where dysphagia is mostly caused by bulky nodal disease or disease within the wall of the esophagus. In such patients an alternative modality such as stenting is preferred.
The primary role of PDT in the past was to treat patients with high-grade dysplasia and Barrett’s esophagus. However, the role of PDT for this indication has been largely replaced by radiofrequency ablation which has been found to be effective and associated with significantly less morbidity (4). PDT is also approved by the Food and Drug Administration for palliative treatment of symptomatic obstructive esophageal cancer. Multiple studies have shown PDT to be effective in alleviating dysphagia, as well as the control of bleeding. In a study of 215 patients with symptomatic or recurrent esophageal cancer, Litle et al. used PDT for palliation of obstruction/bleeding from esophageal cancer. This study also included patients with endoluminal recurrence after esophagectomy. PDT led to improvement of dysphagia in 85% of the patients, and achieved dysphagia free survival of 2 months, and overall survival of 4.8 months. PDT also controlled all esophageal bleeding (6 patients) without the need for additional treatment courses (5).
PDT, for the most part, is a safe procedure with reported serious morbidity and mortality rates between 1.3–3.9% (6,7). Common complications from PDT can include esophageal stricture, and in some cases, esophageal perforation and esophageal fistula formation. Strictures are related to PDT effects on more normal mucosa and so are more of an issue after treatment of high-grade dysplasia. Esophageal dilation is often performed when performing tumor debridement, so care should be taken to minimize excessive dilation. In the Litle et al.’s study, the authors reported common complications after receiving PDT to include sun burns (10%), esophageal strictures (4.8%), fungal esophagitis (3.2%), and symptomatic pleural effusion (3.2%) (5).
Although there is selective accumulation of the photosensitizer within cancer cells, all cells to varying degrees absorb the molecule, including skin. Patients who have received the photosensitizer must avoid sun exposure to minimize skin photosensitivity. Patients are advised to wear gloves, long sleeve shirts, pants, and sunglasses while outdoors and avoid sitting directly next to windows while indoors. This photosensitivity can last up to 30 days for first generation photosensitizer (porfimer sodium), and 2 weeks for second generation photosensitizers (talaporfin sodium) (3,8). Use of second generation photosensitizers has been reported from centers outside of North America (8,9). These have been shown to have better short-term outcomes, with less complication rates overall when compared to first generation photosensitizers as salvage treatment for locally advanced esophageal cancer (9).
Lindenmann et al. reviewed multimodal palliative treatment of esophageal cancer in 250 patients between 1999 and 2009. Treatment modalities used in the study included PDT, stenting, dilation, endoluminal brachytherapy, feeding enterotomy, external radiation, chemotherapy, and palliative resection, with the majority of patients receiving more than one treatment modality. PDT was chosen to be the initial treatment modality in patients without gross tumor infiltration into mediastinal structures due to its ability to quickly relieve symptoms of dysphagia. Overall, 171 patients received PDT treatment, 118 as their initial treatment modality with mean survival being 34 months. When used as the initial modality, PDT was shown to have the most favorable median survival at 50.9 months compared to 17.3 months when other treatments were used as initial modality, although this is likely due to patient selection bias. Major complications after PDT in this study included esophageal perforation, with a rate of 8.8% within 5 days after treatment, and all were localized and controlled with esophageal stenting. Rate of tumor-necrosis related hemorrhage was 7.6%, and all were successfully treated with endoscopy and argon beam coagulation (10).
Cryotherapy
Cryotherapy is a relatively new technique that was initially used to treat Barrett’s esophagus and high grade dysplasia of the esophagus (11). Multiple retrospective studies and case reports suggest that cryotherapy is safe and effective for palliation of esophageal cancer as well. Under endoscopic guidance, a cryoablation catheter is passed through the accessory channel of the endoscope and directed towards the area of ablation. Low pressure liquid nitrogen is then sprayed onto the tumor for 20–40 seconds. The tissue is allowed to thaw, and then refrozen for another 20–40 secs. The cycle may be repeated 2–4 times during each treatment session. Liquid nitrogen will change into a gaseous state after this is released from the catheter. This change from liquid to gas results in about a 700-fold increase in its volume, so there is a risk of luminal perforation. To minimize this, the stomach and esophagus is vented with a specially designed decompression tube (similar to a nasogastric tube). A member of the treatment team will also palpate the abdomen during a treatment to assess for abdominal distension. Follow up sessions may be performed at 2–6 weekly intervals (3,12). These freeze-thaw cycles cause intracellular disruptions and ischemia, leading to tumor destruction. One of the challenges of cryotherapy is that it can be difficult to place a decompression tube alongside an endoscope, and then to clearly visualize anatomy when treating a very bulky tumor. In these instances, an alternative approach such as PDT or stenting may be preferable, although an advantage with cryotherapy is that this avoids the issue of photosensitivity seen with PDT, and the risk of strictures is also lower.
Although this therapy can be repeated multiple times, clinical effects can be seen after a single session. In a study by Shah et al., 21 patients with dysphagia from metastatic or locally advanced esophageal cancers received a single session of cryotherapy. A proportion of 71 of patients had improvement in dysphagia symptoms within 1 week without serious adverse events reported. Furthermore, of the patients with locally advanced esophageal cancer who went on to receive chemoradiation, 67% of patients saw locally complete response and 56% saw complete clinical response, suggesting synergistic effects of combination cryotherapy and chemoradiation (12).
In a retrospective, multicenter study by Kachammy et al., 49 patients with inoperable esophageal cancer with symptoms of dysphagia underwent palliative endoscopic cryotherapy of the entire tumor length including onto the gastroesophageal junction. Sessions were repeated every 2–12 weeks based on severity of dysphagia and response to treatment. A total of 120 cryotherapy sessions were performed for 49 patients and 65% of sessions occurring with concomitant chemotherapy. Dysphagia scores (Likert Scale) were available for 113 cryotherapy sessions. 59.3% of sessions demonstrated improvement in dysphagia scores, 38.1% of sessions resulted in no improvement, and 2.7% of sessions led to worsening of dysphagia by a single point. Those who did not demonstrate improvement of dysphagia with cryotherapy underwent alternative treatment modalities such as esophageal dilation or stenting. Five percent of cryotherapy sessions were associated with minor adverse effects such as chest pain, and 1 patient developed postprocedural stricture which required endoscopic dilation (13).
Adverse side effects associated with cryotherapy are infrequent and often minor. In a study investigating safety and effectiveness of cryotherapy, Tsai et al. recruited 88 patients with esophageal cancer across 11 centers for either curative or palliative cryotherapy. All had refused, failed, or were considered not appropriate for conventional therapy such as esophagectomy, chemotherapy, or radiation therapy. A total of 359 cryotherapy treatments were administered without treatment related perforations or deaths. Twelve (13.6%) patients developed strictures, however, 3 of those patients had strictures before cryotherapy. Other commonly reported post-procedure adverse side effects included abdominal pain (19.3%), dysphagia (10.2%), sore throat (9%) and chest pain (8%). Complete eradication of luminal tumors was achieved in 55.8% of patients, and 44.2% of patients had incomplete response to therapy (14). In another respective study by Greenwald et al., 79 patients from 10 institutions with esophageal carcinoma who have failed or were ineligible for conventional therapy received cryotherapy. Tumor stage ranged from T1 to T3 and tumor length raged from 1–15 cm. Complete response was achieved in 61.2% of patients. No serious adverse events, including perforation and hemorrhage, were reported. Benign strictures were noted in 10 patients, all of whom have received previous treatments such as resection, PDT and chemotherapy. Posttreatment pain requiring narcotics were reported in 27% patients (15). Overall, cryotherapy was found to be a well-tolerated and an efficacious treatment for esophageal cancer either as an adjunct or alternative to conventional therapy.
YAG laser
There remains relatively little data regarding the utility of Nd:YAG laser therapy (YAG) for malignant dysphagia. Comparison of laser ablation with expandable metal stent placement for dysphagia has been studied in randomized controlled trials with data demonstrating superiority in the thermal ablation group in regard to quality of life. Relief of dysphagia symptoms appear to be poor in either group studied (16). Comparative studies of YAG and PDT demonstrate superior results for PDT regarding luminal patency for tumors greater than 8 cm, tumors located in the upper third of the esophagus, and circumferential lesions. In one prospective multicenter randomized trial by Lightdale et al., 218 patients with advanced esophageal cancer were evenly randomized to receive either PDT or YAG. Initial improvements in dysphagia were equivalent between groups, however at 1 month evaluation, 32% of patients in the PDT treatment arm remained asymptomatic in comparison to 20% in the YAG treatment arm. Nine patients in the PDT group demonstrated complete tumor response, versus two after YAG (17,18). A disadvantage of YAG laser treatment is that esophageal tumors often run over several centimeters of the length of the esophagus. YAG laser fibers are end-diffuser fibers so this is more of a point-and-shoot therapy, requiring precise energy delivery at the end of the fiber. The risk of perforation is potentially higher, and by necessity these procedures are longer than that required for PDT or cryotherapy where several centimeters of esophageal tumor can be treated at the same time. It can be concluded that although YAG therapy has been utilized in tumor ablation, its use for palliation in esophageal cancer for symptoms of dysphagia demonstrates inferiority when compared to PDT.
Argon plasma coagulation (APC)
APC has been described in treatment of Barrett’s esophagus and in small studies and case reports for tumor debulking. However, its role in esophageal cancer remains unclear. APC has been described for alleviation of in-stent tumor growth and control of bleeding utilizing non-contact electrocoagulation, however data is unclear regarding palliative treatment of dysphagia, symptom recurrence, complications, and effects on mortality (19). In a randomized comparison study by Rupinski et al., APC monotherapy was compared to APC in combination with high dose rate (HDR) brachytherapy and APC in combination with PDT to determine benefit of these additional therapies to APC for treatment of malignant dysphagia. This study concluded significant improvement for time until first dysphagia recurrence in the combination groups than APC monotherapy (20).
Summary
The unfortunate reality of esophageal cancer is its advanced stage at presentation in majority of patients. Endoluminal techniques are evolving for the palliation of symptoms of dysphagia including many techniques used previously for Barrett’s esophagus and high-grade dysplasia. The most frequently used modalities include stent placement, dilation, PDT, and cryotherapy, with the latter two being the most common ablative techniques reported for palliation.
Although PDT is limited due to its significant phototoxicity, use of second-generation agents utilizing the same basic principles and efficacy may allow PDT to regain popularity as a palliation alternative. PDT has been demonstrated to be efficacious at relieving obstruction and controlling endoluminal bleeding but multiple treatment courses may be required. Cryotherapy technique also demonstrates efficacious results for relief of dysphagia, and additionally has the main advantage of avoiding the photosensitivity associated with PDT. Another advantage of cryotherapy is that this avoids the need for a several-day treatment course (i.e., injection with repeated endoscopies) as seen with PDT. However, cryotherapy can be challenging to perform when treating a bulky tumor.
While not discussed here, stenting remains a viable alternative that is arguably the mainstay of palliation in most centers. Stenting is relatively simple to perform and is a “one and done” technique without the need for additional endoscopies or treatment. However, stenting is not without issue. Some patients will complain of significant discomfort related to the radial expansion of stents within the mediastinum, and in some cases will require stent removal. Stents may migrate, may become obstructed with food, and may be associated with tumor over-growth at the ends of the stent and tumor in-growth through the interstices of the stent if an uncovered stent is used. Ablative techniques can be very helpful when tumor in-growth or over-growth occurs. Another issue is that stents that cross the gastroesophageal junction can be associated with significant reflux and a risk of aspiration. Stents that are close to the cricopharyngeus can be associated with a globus sensation. In some patients with bulky cervical tumors, deployment of a stent can lead to airway compression. In such patients, endoscopic ablation will be preferred.
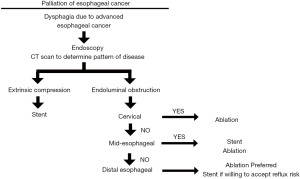
Physicians treating esophageal cancer should be familiar with and have different options available. Although malignant dysphagia contributes significantly to patient discomfort, other aspects of palliation, including quality of life, need to be assessed prior to addressing a single portion of the disease process; palliative treatment for dysphagia are not without complications. Figure 2 suggests a decision-tree that can be applied for endoscopic palliation of dysphagia with the reality that a multi-modal or multi-session approach may need to be undertaken to get optimal results.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Abbas E. Abbas and Roman V. Petrov) for the series “New Technologies in Esophageal Surgery and Endoscopy” published in Annals of Esophagus. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-51/rc
Peer Review File: available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-51/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-51/coif). The series “New Technologies in Esophageal Surgery and Endoscopy” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Surveillance Research Program (SRP) in NCI's Division of Cancer Control and Population Sciences (DCCPS). Cancer of the Esophagus - Cancer Stat Facts. SEER. Available online: 2021. https://seer.cancer.gov/statfacts/html/esoph.html
- Overholt BF, Wang KK, Burdick JS, et al. Five-year efficacy and safety of photodynamic therapy with Photofrin in Barrett's high-grade dysplasia. Gastrointest Endosc 2007;66:460-8. [Crossref] [PubMed]
- Gurmendi AF, Fernando HC. Chapter 29: Esophageal Cancer: Alternative Energy Source in Treatment. In: Fezza EE, Gagner M, Li MKW. editors. International Principles of Laparoscopic Surgery. 1st ed. Cine-Med, Inc., 2009:267-75.
- Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett's esophagus with dysplasia. N Engl J Med 2009;360:2277-88. [Crossref] [PubMed]
- Litle VR, Luketich JD, Christie NA, et al. Photodynamic therapy as palliation for esophageal cancer: experience in 215 patients. Ann Thorac Surg 2003;76:1687-92; discussion 1692-3. [Crossref] [PubMed]
- McCaughan JS Jr, Ellison EC, Guy JT, et al. Photodynamic therapy for esophageal malignancy: a prospective twelve-year study. Ann Thorac Surg 1996;62:1005-9; discussion 1009-10. [Crossref] [PubMed]
- Gilbert S, Luketich JD, Fernando HC. Endoscopic therapies for the airway and the esophagus. In: Selke FW, delNido PJ, Swanson SJ. editors. Surgery of the Chest. Philadelphia, Pa: WB Saunders, 2004:65-78.
- Minamide T, Yoda Y, Hori K, et al. Advantages of salvage photodynamic therapy using talaporfin sodium for local failure after chemoradiotherapy or radiotherapy for esophageal cancer. Surg Endosc 2020;34:899-906. [Crossref] [PubMed]
- Yano T, Kasai H, Horimatsu T, et al. A multicenter phase II study of salvage photodynamic therapy using talaporfin sodium (ME2906) and a diode laser (PNL6405EPG) for local failure after chemoradiotherapy or radiotherapy for esophageal cancer. Oncotarget 2017;8:22135-44. [Crossref] [PubMed]
- Lindenmann J, Matzi V, Neuboeck N, et al. Individualized, multimodal palliative treatment of inoperable esophageal cancer: clinical impact of photodynamic therapy resulting in prolonged survival. Lasers Surg Med 2012;44:189-98. [Crossref] [PubMed]
- Shaheen NJ, Greenwald BD, Peery AF, et al. Safety and efficacy of endoscopic spray cryotherapy for Barrett's esophagus with high-grade dysplasia. Gastrointest Endosc 2010;71:680-5. [Crossref] [PubMed]
- Shah T, Kushnir V, Mutha P, et al. Neoadjuvant cryotherapy improves dysphagia and may impact remission rates in advanced esophageal cancer. Endosc Int Open 2019;7:E1522-7. [Crossref] [PubMed]
- Kachaamy T, Prakash R, Kundranda M, et al. Liquid nitrogen spray cryotherapy for dysphagia palliation in patients with inoperable esophageal cancer. Gastrointest Endosc 2018;88:447-55. [Crossref] [PubMed]
- Tsai FC, Ghorbani S, Greenwald BD, et al. Safety and efficacy of endoscopic spray cryotherapy for esophageal cancer. Dis Esophagus 2017;30:1-7. [Crossref] [PubMed]
- Greenwald BD, Dumot JA, Abrams JA, et al. Endoscopic spray cryotherapy for esophageal cancer: safety and efficacy. Gastrointest Endosc 2010;71:686-93. [Crossref] [PubMed]
- Dallal HJ, Smith GD, Grieve DC, et al. A randomized trial of thermal ablative therapy versus expandable metal stents in the palliative treatment of patients with esophageal carcinoma. Gastrointest Endosc 2001;54:549-57. [Crossref] [PubMed]
- Marcon NE. Photodynamic therapy and cancer of the esophagus. Semin Oncol 1994;21:20-3. [PubMed]
- Lightdale CJ, Heier SK, Marcon NE, et al. Photodynamic therapy with porfimer sodium versus thermal ablation therapy with Nd:YAG laser for palliation of esophageal cancer: a multicenter randomized trial. Gastrointest Endosc 1995;42:507-12. [Crossref] [PubMed]
- Akhtar K, Byrne JP, Bancewicz J, et al. Argon beam plasma coagulation in the management of cancers of the esophagus and stomach. Surg Endosc 2000;14:1127-30. [Crossref] [PubMed]
- Rupinski M, Zagorowicz E, Regula J, et al. Randomized comparison of three palliative regimens including brachytherapy, photodynamic therapy, and APC in patients with malignant dysphagia (CONSORT 1a) (Revised II). Am J Gastroenterol 2011;106:1612-20. [Crossref] [PubMed]
Cite this article as: Zhao D, Shahbahrami K, Fernando HC. Endoscopic palliation of esophageal cancer: a narrative review apart from stenting. Ann Esophagus 2023;6:22.