
Endoscopic tissue approximation in clinical practice and the OverStitch device: a narrative review
Introduction
Endoscopic clips and suturing devices have evolved over the past 15 years to have a wide range of applications including closure of perforations, leaks, fistulas, mucosal defects after resections, anti-reflux and weight loss procedures and others. These advancements in endoscopic technologies and devices have allowed for innovations in the management of a wide variety of esophageal and foregut conditions. We present the following article in accordance with the Narrative Review reporting checklist (available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-50/rc).
Overview of currently available devices
Through the scope clips
Various through the scope endoscopic clips have allowed for control of gastrointestinal bleeding, and approximation of mucosal injuries and full thickness defects (Figure 1, Video 1: 0:09, 1:22). The use of endoscopic clips was initially described over 30 years ago (1). Since then, the variety and functionality of modern-day clips has progressed exponentially. Most of the modern through the scope clips can be used in an endoscope with a 2.8-mm working channel, which is the majority of the diagnostic endoscopes on the market. These clips can be used as an effective monotherapy for hemostasis, when compared to injections or thermal coagulation (1,2). Overall, through the scope clips allow for excellent field exposure and visualization due to their compact size, and can be used in most commercially available endoscopes. Of the multitude of the products available, in our practice we prefer Resolution 360TM (Boston Scientific, Marlborough, MA, USA) clip. In authors opinion this particular device has superior performance due to precise 1:1 control of the rotational position of the distal tip (3). The Resolution 360 clip has been demonstrated to have 100% reliability when fired on a 10-mm gel, simulating firing across dense fibrinous tissue such as an ulcer.

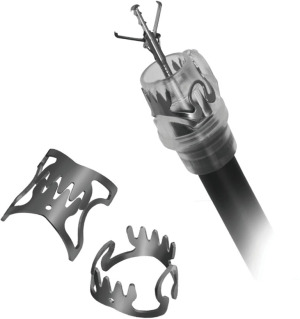
Over the scope clip system (Ovesco Clip)
The Ovesco Clip (Ovesco Endoscopy AG, Tubingen, Germany) is an over-the-scope nitinol clip, widely known as a “bear claw” or “bear trap” for its characteristic appearance (Figure 2). Nitinol is a biocompatible metal alloy, with unique properties, particularly shape memory upon heating. The clip was FDA approved for use in the US in 2010. The system is composed of an applicator cap that the clip is mounted on, a thread and thread retriever, and a hand wheel that releases the clip. Once the target lesion is identified, it is suctioned into the cap. The thread is tightened with the hand wheel, which closes the clip system and helps anchor the tissue. Once it is determined that all the target tissue is pulled into the cap, the clip can be deployed. There are three types of clip tooth shapes—traumatic (used to close fistulas, perforations), atraumatic (used for controlling bleeding), gastrostomy closure, and caps of different depths that allow for grasping tissue during the approximation (3,4).
OverStitch Endoscopic Suturing System
The OverStitch Endoscopic Suturing System (Apollo Endosurgery, Austin, TX, USA) was initially developed in 2009, and FDA approved in 2010 (Figure 3). An updated version of the device was released in 2011, fitting only double channel Olympus therapeutic endoscope. In 2018 the new generation OverStitch SX was launched, featuring the ability to use the device with a single-channel endoscope, allowing compatibility with all four commercially available platforms (Olympus, Pentax, Fuji and Storz). While more expensive and more time consuming compared to through or over the scope clips, the OverStitch device provides classical surgical suturing, potentially offering better approximation of larger defects, or defects with irregular edges (5,6).
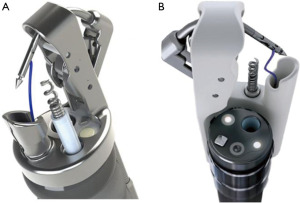
The components of the device include an end cap, that is mounted on the end of the scope, a needle driver handle, an anchor exchange catheter and a tissue retraction helix device that assists in approximating the tissue. The original OverStitch device is mounted on the distal end of a double-channel therapeutic endoscope, while the OverStitch SX works with a single-channel endoscopes, essentially transitioning the working channel to externally attached components (5).
The suture is mounted on the exchange catheter, and passed down through the operating channel, where it is transferred to the needle driver. The helix device is then anchored in and used to retract the target tissue back into the device. The needle driver handle is then closed, passing the anchor and suture through the grasped tissue. The anchor exchange catheter is advanced over the anchor, and locked into place. The anchor exchange catheter is retracted, pulling the anchor exchange catheter away from the needle body, and then the tissue is released from the device by releasing the helix device. The needle driver handle is then released, leaving a full thickness suture placement. These steps can be repeated for further closure of defects with interrupted or continuous stitches. The suture is closed by releasing the anchor exchange catheter, which disengages the anchor from the device. The cinch catheter is then advanced, and tension pulled on the suture, closing the defect. Next, the cinch is deployed, and the suture locked in place and cut. The design of the device allows for multiple reloads without having to remove the endoscope (6) (Video 1: 2:36, 4:10, 5:19).
X-Tack
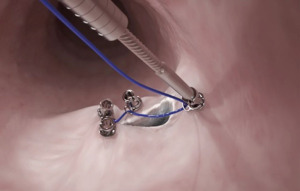
The X-Tack (Apollo Endosurgery, Austin, TX) is a newly developed through-the-scope suturing tool that allows for closure of tissue defects with nearly surgical suturing via the endoscope channel (Figure 4). The novel device uses helical coil tissue tacks that are pre-strung on a suture, and can be placed along the edge of the resected tissue, then cinched together to achieve tissue re-approximation. The main benefits of this device are that it allows for closure of defects in areas difficult to reach with larger devices, and it allows for immediate use without extraction of the endoscope for installation of the suturing device (7).
Applications of the tissue approximation and suturing devices
Full thickness gastrointestinal defects
Full thickness gastrointestinal defects can be categorized as either acute or non-acute. Acute defects can occur iatrogenically, such as perforations during endoscopic procedures, spontaneously, in cases of malignancies, ulcers, or Boerhaave syndrome or traumatically, from foreign bodies, blunt and penetrating trauma (4). Non-acute defects can occur from leaks - due to anastomotic breakdown, infections or inflammation. Over time leaks can develop epithelialized tracts, resulting in fistulas. Leaks are a known and feared complication following esophagectomies, that can result in a prolonged hospital stay, require a return to the operating room and increase mortality (4,8). Management of these complications is complex, including the need for long-term antibiotics, nutrition optimization and further temporizing measures. Surgical resections are often challenging, due to infected and friable tissue from long-term inflammation. The high morbidity of these open repairs is the driving force behind the numerous endoscopic interventions that have been recently developed.
Management of acute defects
Endoscopic repair of acute full thickness defects has been well established in the literature. The use of over the scope clips was analyzed in a review study of 24 publications and 466 acute injuries initially managed by endoscopic closure (9). In 419 (90%) cases successful closure of the defect was achieved. A single adverse event was reported with an additional full thickness injury during the endoscopic intervention. This analysis has demonstrated over-the-scope clipping as a safe alternative to surgery in patients with acute full thickness gastrointestinal tract defects. Previous studies have also demonstrated the efficacy of through the scope clipping in management of smaller perforations <10 mm, with a 100% success rate across several case reports (10-12) (Video 1: 0:09).
Management of non-acute defects
Endoscopic suturing techniques have allowed for further minimally invasive management of leaks and fistulas, providing surgical suture closure of the defects, mimicking that of the open procedures via endoscopic approach (13,14) (Video 1: 1:22). Chon et al. studied the management of upper gastrointestinal (UGI) leaks with endoscopic suturing with the OverStitch device (15). The authors reviewed 13 patients with postoperative leaks, five of whom had previous failed attempts at leak closure with endoscopic clipping, stenting or vacuum therapy. They demonstrated 100% success in leak management in patients who had not had prior attempts at leak management (61.5%). In patients who had previously undergone endoscopic treatments, the procedure was unsuccessful, requiring further intervention for correction of the leaks (38.5%) (15). Mukewar et al. reviewed 56 patients who underwent fistula closure with the OverStitch device, and presented initial technical success in 100% of cases, with 17 (30%) patients requiring delayed repeat endoscopic intervention. These repeat interventions included additional endoscopic suturing procedures and six patients required surgery (16). In another study of 97 patients with either fistulas or leaks, Morrell et al. achieved 66% successful closure at 6 months, with only 15% of patients requiring surgical intervention (13).
Stent anchoring
Covered self-expanding esophageal stents are used in the palliation of malignant strictures, and in the treatment of esophageal perforations, leaks and fistulas. A common complication of this procedure is stent migration, occurring in up to 40% of cases (17). Whereas placement of clips and snares has been reported for stent fixation, it is technically challenging and has a high rate of complications. Endoscopic suturing has emerged as the preferred method of stent fixation (Video 1: 2:36). Kantsevoy et al. reviewed 7 patients in whom the OverStitch device was used for correcting migrated stents, or prophylactically during initial stent placement. In both groups there was no stent migration after suture fixation (18). A comparison study in patients with locally advanced esophageal cancer demonstrated over three times lower migration rates in the group with suture fixation (8% vs. 27%) (19). Similarly, Ngamruengphong et al. in the analysis of 125 patients with benign UGI diseases, such as strictures, leaks, fistulas and perforations, showed decrease in the migration rate to 16% from 33% with the use of suture fixation (20). A meta-analysis by Law et al. identified 14 studies with 212 patients who had placement of self-expandable metal stents with endoscopic suture fixation. They described a 17% migration rate after placement of endoscopic sutures. Migration after placement was identified endoscopically and radiographically. In addition to the significantly lower rates of migration seen in the endoscopic suture fixation group, those patients had a longer time to stent migration resulting in better clinical outcomes, and overall required fewer procedures (21).
Endoscopic suturing in the management of gastroesophageal reflux disease (GERD)
Rising incidence of GERD propelled this problem into the forefront of the healthcare focus. Whereas medical therapy remains a gold standard for initial intervention, in patients who fail medical treatment, anti-reflux surgery is the next step. Increasing focus on minimally invasive approaches lead to the development of several endoscopic procedures for the management of GERD. There is little data available on the utility of the OverStitch device in the surgical management of GERD. However, several publications allude to the utility of endoscopic suturing in post-esophagectomy reflux. Reflux after radical esophagectomy, called gastric tube-esophageal reflux (GTER) is a well-known condition that significantly affects quality of life and leads to various complications (22). A small animal study demonstrated the feasibility of using the OverStitch device in a post-esophagectomy pig model to create an anti-reflux valve. The device was used to create a funnel using a posterior plication method in a series of four animals. Effectiveness of the funnel was evaluated using water soluble contrast to determine a reflux angle, which was higher after funnel creation in all cases, and a pH monitoring, which showed a stable pH compared to significant reflux seen before the procedure. The authors reported a median operating time of 43 minutes, and saw no post-operative adverse events (23). Nagase et al. replicated the procedure in a 69-year-old male esophageal cancer survivor who was 10 years from his initial resection and suffering from severe GTER. They reported improvements in postoperative pH control, and the patient reported significant improvements in his quality of life (22).
Applications in peroral endoscopic myotomy
Achalasia is the most common esophageal motility disorder. The etiology is believed to be a neurodegenerative process of the vagus nerve and myenteric plexus, resulting in failure of lower esophageal sphincter relaxation and loss of esophageal peristalsis. High-resolution manometry (HRM) is a gold standard diagnostic test, allowing further subdivision of the condition into three subtypes (24-26).
Pneumatic balloon dilation and surgical cardiomyotomy are gold standard interventions for management of achalasia (27,28). In the last decade, perioral endoscopic myotomy (POEM) has emerged as a safe, minimally invasive technique used in the treatment of these patients. The procedure involves four distinct steps—mucosotomy, submucosal tunneling, myotomy proper and closure of the mucosotomy. Adequate closure of the mucosotomy at the conclusion of the procedure is crucial for preventing spilling of luminal content into the mediastinum. The mucosotomy at the entry site can be closed with endoscopic clips or the OverStitch device (6,29) (Video 1: 0:09, 4:10).
Pescarus et al. performed a case-controlled study looking at endoclips versus endoscopic suturing in mucosotomy closure during POEMs. The authors found that closure time was significantly shorter with clipping (16±12 vs. 33±11 minutes), and a higher overall cost was associated with suturing (1,502±849 vs. 2,521±575 USD). However, in a case of inadvertent full thickness defect during initial mucosotomy, successful closure was achieved with the OverStitch device. The authors highlight that while the OverStitch device was slower than clipping, it was very useful in cases where the defect was so large that closure with endoscopic clips would have been difficult secondary to the small jaw span of the clips (30). The authors of this article preferentially utilize OverStitch device for the purpose of mucosotomy closure after submucosal procedures due to the watertight nature of the closure. Clips usually utilized as a rescue technique in cases where due to difficult angles adequate exposure and application of suture is challenging or impossible.
Applications in bariatric procedures
Obesity is a serious public health concern affecting 39% of the world’s population, or nearly two billion people worldwide (31). In the US, obesity is the second leading cause of preventable death, and is a major risk factor for heart disease, stroke, diabetes, and several cancers. Whereas bariatric surgery has been shown to be an effective treatment for obesity, complications after bariatric surgery include leaks, perforations and fistulas, reaching 3–20% morbidity and 0.1–0.5% mortality rate (32).
Endoscopic therapies for bariatric weight loss have been shown to be safe and effective in treatment of obesity, as a bridge to surgical treatment, and are becoming a popular treatment choice for patients who do not qualify for bariatric surgery. Endoscopic sleeve gastroplasty (ESG) was developed in 2008 and is similar to a laparoscopic sleeve gastrectomy (LSG) in that it acts to create a restrictive pattern of weight loss (33). During the procedure, the OverStitch system is used to place full thickness stitches along the greater curvature of the stomach from the pre-pyloric antrum to the gastroesophageal junction, creating a restrictive, sleeve-like configuration (33).
Several studies have looked at the efficacy of the OverStitch in ESG. Neto et al. analyzed 233 patients, with a mean baseline body mass index (BMI) of 34.7 who underwent an ESG. Weight loss was 17.1% at 6 months, and 19.7% at 12 months, with only one bleeding complication (33). A study of 91 patients by Sharaiha et al. showed a 14.4% total body weight loss at 6 months, 17.6% at 12 months, and 20.9% at 24 months in a population with a mean pre-procedure BMI of 40.7±7.0 kg/m2. Significant reduction in hemoglobin A1c, systolic blood pressure, alanine aminotransferase and serum triglycerides were also seen, and complications included a perigastric leak that occurred in one patient (34). In the largest study to date, Alqahtani et al. reviewed 1,000 patients with a mean baseline BMI of 33.3 (±4.5) kg/m2 who underwent ESG and were followed for 18 months post-procedurally. Mean total weight loss was 13.7%±16.8% at 6 months, 15%±7.7% at 12 months, and 14.8%±8.5% at 18 months. Complications included readmission in 24 (2.4%), severe abdominal pain in 8 (0.8%) and bleeding in 7 (0.7%) patients, with only 2 (0.2%) of them requiring blood transfusion. Five (0.5%) patients required re-do ESG and 8 (0.8%) patients were revised to a sleeve gastrectomy. As in other publications, significant improvement in hypertension, diabetes and dyslipidemia were seen (35).
Novikov et al. compared ESG, LSG and laparoscopic adjustable gastric banding (LAGB). The authors analyzed 278 patients with a mean baseline BMI of 43.82 (±0.5) kg/m2. Of these, 91 patients underwent ESG, 120 LSG, and 67 had a LAGB. At 6 months weight loss in the LSG group was 23.48% vs. 14.37% in the ESG group and 12.68% in the LAGB group. Weight loss was similar at 12 months, with the greatest in the LSG, followed by ESG and LABG (29.28% vs. 17.57% vs. 13.30%). When stratified by BMI <40 kg/m2, and adjusting for age, gender and American Society of Anesthesiologists (ASA), there was no significant difference in percent total body weight lost at 12 months across all three different procedures. The complication rate in the LSG group was 9.17% and included peri-gastric leaks, wound dehiscence with herniation, postoperative ileus and wound infection requiring antibiotics. The ESG demonstrated lower overall complication rate at 2.20% and included a peri-gastric leak, while the rate in LAGB complications was 8.96% and included band removal due to obstruction, abdominal pain, and a wound infection (36).
Conclusions
Significant progress in the spectrum of diseases successfully managed with endoscopic interventions is predicated upon refinement of endoscopic technologies and instrumentation. More advanced procedures, like resection of the neoplasms, closure of fistulas and perforation, and even anatomical interventions, such as anti-reflux valves or gastroplasty are dependent on the advanced techniques of tissue approximation. Available through the scope or over the scope clipping and suturing instruments have facilitated astonishing growth in the field. Future developments and refinements of the technology are likely to result in many currently common surgical procedures becoming obsolete and being replaced by endoscopic interventions. Surgeons, especially foregut specialists, need to stay abreast of the developments to retain expertise and leadership roles in this rapidly evolving field.
Acknowledgments
Funding: This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA006927.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Esophagus for the series “New Technologies in Esophageal Surgery and Endoscopy”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-50/rc
Peer Review File: Available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-50/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-50/coif). The series “New Technologies in Esophageal Surgery and Endoscopy” was commissioned by the editorial office without any funding or sponsorship. AA served as an unpaid Guest Editor of the series and serves as an unpaid editorial board member of Annals of Esophagus from May 2020 to April 2022. PP served as an unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wellington J, Canakis A, Kim R. Endoscopic closure devices: A review of technique and application for hemostasis. Int J Gastrointest Interv 2019;8:127-33. [Crossref]
- ASGE Standards of Practice Committee. The role of endoscopy in the management of suspected small-bowel bleeding. Gastrointest Endosc 2017;85:22-31. [Crossref] [PubMed]
- Kobara H, Mori H, Nishiyama N, et al. Over-the-scope clip system: A review of 1517 cases over 9 years. J Gastroenterol Hepatol 2019;34:22-30. [Crossref] [PubMed]
- Winder JS, Pauli EM. Comprehensive management of full-thickness luminal defects: The next frontier of gastrointestinal endoscopy. World J Gastrointest Endosc 2015;7:758-68. [Crossref] [PubMed]
- Stavropoulos SN, Modayil R, Friedel D. Current applications of endoscopic suturing. World J Gastrointest Endosc 2015;7:777-89. [Crossref] [PubMed]
- Kantsevoy SV. The Development of the Overstitch System and Its Potentials. Gastrointest Endosc Clin N Am 2020;30:107-14. [Crossref] [PubMed]
- Hernandez A, Marya NB, Sawas T, et al. Gastrointestinal defect closure using a novel through-the-scope helix tack and suture device compared to endoscopic clips in a survival porcine model (with video). Endosc Int Open 2021;9:E572-7. [Crossref] [PubMed]
- Geraci G, Pisello F, Modica G, et al. Complications of elective esophago-gastro-duodenoscopy (EGDS). Personal experience and literature review. G Chir 2009;30:502-6. [PubMed]
- Verlaan T, Voermans RP, van Berge Henegouwen MI, et al. Endoscopic closure of acute perforations of the GI tract: a systematic review of the literature. Gastrointest Endosc 2015;82:618-28.e5. [Crossref] [PubMed]
- Yılmaz B, Unlu O, Roach EC, et al. Endoscopic clips for the closure of acute iatrogenic perforations: Where do we stand? Dig Endosc 2015;27:641-8. [Crossref] [PubMed]
- Biancari F, Saarnio J, Mennander A, et al. Outcome of patients with esophageal perforations: a multicenter study. World J Surg 2014;38:902-9. [Crossref] [PubMed]
- Biancari F, Gudbjartsson T, Mennander A, et al. Treatment of esophageal perforation in octogenarians: a multicenter study. Dis Esophagus 2014;27:715-8. [Crossref] [PubMed]
- Morrell DJ, Winder JS, Johri A, et al. Over-the-scope clip management of non-acute, full-thickness gastrointestinal defects. Surg Endosc 2020;34:2690-702. [Crossref] [PubMed]
- Ge PS, Thompson CC. The Use of the Overstitch to Close Perforations and Fistulas. Gastrointest Endosc Clin N Am 2020;30:147-61. [Crossref] [PubMed]
- Chon SH, Toex U, Plum PS, et al. Efficacy and feasibility of OverStitch suturing of leaks in the upper gastrointestinal tract. Surg Endosc 2020;34:3861-9. [Crossref] [PubMed]
- Mukewar S, Kumar N, Catalano M, et al. Safety and efficacy of fistula closure by endoscopic suturing: a multi-center study. Endoscopy 2016;48:1023-8. [Crossref] [PubMed]
- Rieder E, Dunst CM, Martinec DV, et al. Endoscopic suture fixation of gastrointestinal stents: proof of biomechanical principles and early clinical experience. Endoscopy 2012;44:1121-6. [Crossref] [PubMed]
- Kantsevoy SV, Bitner M. Esophageal stent fixation with endoscopic suturing device (with video). Gastrointest Endosc 2012;76:1251-5. [Crossref] [PubMed]
- Yang J, Siddiqui AA, Kowalski TE, et al. Esophageal stent fixation with endoscopic suturing device improves clinical outcomes and reduces complications in patients with locally advanced esophageal cancer prior to neoadjuvant therapy: a large multicenter experience. Surg Endosc 2017;31:1414-9. [Crossref] [PubMed]
- Ngamruengphong S, Sharaiha RZ, Sethi A, et al. Endoscopic suturing for the prevention of stent migration in benign upper gastrointestinal conditions: a comparative multicenter study. Endoscopy 2016;48:802-8. [Crossref] [PubMed]
- Law R, Prabhu A, Fujii-Lau L, et al. Stent migration following endoscopic suture fixation of esophageal self-expandable metal stents: a systematic review and meta-analysis. Surg Endosc 2018;32:675-81. [Crossref] [PubMed]
- Nagase H, Yamasaki M, Yanagimoto Y, et al. Successful Endoscopic Treatment of Post-esophagectomy Refractory Reflux Using OverStitch: The First Clinical Case. Clin Med Insights Gastroenterol 2018;11:1179552218784946. [Crossref] [PubMed]
- Yanagimoto Y, Yamasaki M, Nagase H, et al. Endoscopic anti-reflux valve for post-esophagectomy reflux: an animal study. Endoscopy 2016;48:1119-24. [Crossref] [PubMed]
- Schlottmann F, Herbella FA, Patti MG. Understanding the Chicago Classification: From Tracings to Patients. J Neurogastroenterol Motil 2017;23:487-94. [Crossref] [PubMed]
- Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil 2015;27:160-74. [Crossref] [PubMed]
- Fajardo RA, Petrov RV, Bakhos CT, et al. Endoscopic and Surgical Treatments for Achalasia: Who to Treat and How? Gastroenterol Clin North Am 2020;49:481-98. [Crossref] [PubMed]
- Khashab MA, Messallam AA, Onimaru M, et al. International multicenter experience with peroral endoscopic myotomy for the treatment of spastic esophageal disorders refractory to medical therapy (with video). Gastrointest Endosc 2015;81:1170-7. [Crossref] [PubMed]
- Petrov RV, Fajardo RA, Bakhos CT, et al. Peroral endoscopic myotomy: techniques and outcomes. Shanghai Chest 2021;5:14. [Crossref] [PubMed]
- Kurian AA, Bhayani NH, Reavis K, et al. Endoscopic suture repair of full-thickness esophagotomy during per-oral esophageal myotomy for achalasia. Surg Endosc 2013;27:3910. [Crossref] [PubMed]
- Pescarus R, Shlomovitz E, Sharata AM, et al. Endoscopic suturing versus endoscopic clip closure of the mucosotomy during a per-oral endoscopic myotomy (POEM): a case-control study. Surg Endosc 2016;30:2132-5. [Crossref] [PubMed]
- World Health Organization. Overweight and obesity. WHO. 2021, March 19. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
- Kassir R, Debs T, Blanc P, et al. Complications of bariatric surgery: Presentation and emergency management. Int J Surg 2016;27:77-81. [Crossref] [PubMed]
- Neto MG, Moon RC, de Quadros LG, et al. Safety and short-term effectiveness of endoscopic sleeve gastroplasty using overstitch: preliminary report from a multicenter study. Surg Endosc 2020;34:4388-94. [Crossref] [PubMed]
- Sharaiha RZ, Kumta NA, Saumoy M, et al. Endoscopic Sleeve Gastroplasty Significantly Reduces Body Mass Index and Metabolic Complications in Obese Patients. Clin Gastroenterol Hepatol 2017;15:504-10. [Crossref] [PubMed]
- Alqahtani A, Al-Darwish A, Mahmoud AE, et al. Short-term outcomes of endoscopic sleeve gastroplasty in 1000 consecutive patients. Gastrointest Endosc 2019;89:1132-8. [Crossref] [PubMed]
- Novikov AA, Afaneh C, Saumoy M, et al. Endoscopic Sleeve Gastroplasty, Laparoscopic Sleeve Gastrectomy, and Laparoscopic Band for Weight Loss: How Do They Compare? J Gastrointest Surg 2018;22:267-73. [Crossref] [PubMed]
Cite this article as: Miller C, Magarinos J, Akcelik A, Bakhos C, Abbas A, Petrov R. Endoscopic tissue approximation in clinical practice and the OverStitch device: a narrative review. Ann Esophagus 2023;6:21.


