
Relay therapy with endovac and endoscopic stents for anastomotic leaks after minimally invasive esophagectomy
Introduction
Esophagectomy with gastric pull up is the most commonly surgical treatment performed for early stage and loco-regionally advanced esophageal cancer (1). Although minimally invasive esophagectomy (MIE) improve postoperative outcomes, regardless of the esophagectomy technique and the type of reconstruction, this procedure is associated with significant morbidity rate.
Esophagogastric anastomosis leak (EAL) is one of the most frequent and feared complications after esophagectomy (2-4), leading to prolonged length of intensive care and hospital stay, substantial cost to the patient and hospital, increased postoperative mortality, and reduced quality of life (5,6). Despite the major improvements in surgical techniques and devices, the incidence of this complication has remained stable and ranges from 5% to 30% (7-9).
While there is a broad therapeutic spectrum from conservative endoscopic to surgical options, a precise therapeutic algorithm has not been clearly established. However, endoscopy has widely gained acceptance and has significantly decreased the morbidity and mortality compared to the surgical approach (10).
Endoscopic fully covered self-expanding metal stents (FSEMS) have been considered as an effective tool for postsurgical esophageal leakage, with a clinical success rate ranging from 44% to over 88% (11-14). However, several studies have reported several problems associated with the use of stents for this indication. The most common include stent migration, difficulty of stent removal owing to tissue ingrowth, and stricture development after stent removal (13-17). Although rarer, mortality due to stent erosion in large mediastinal vessels has been reported.
Endoscopic vacuum therapy (EVT) with a sponge has recently gained acceptance for management of anastomotic failures. Particularly for esophageal leaks, EVT has been reported in several publications to successfully heal esophageal leaks in more than 89% of cases with low mortality rates (18-24).
This endoscopic approach relies on the same principles of the vacuum-assisted closure therapy of external wounds [i.e., improvement and accelerated healing by removing infected secretions, reducing edema, increasing local perfusion, and promoting granulation tissue formation (25-28)]. The main limitation for this approach has been the number of procedures required in long healing defects.
Combination of both therapies together for complex leakages has been reported as Stent-over-sponge (SOS) technique (29). The aim of that combination is to ensure sponge adherence to the underlying tissue, optimizing suction direction and efficacy. SOS was indicated for the treatment of uncontained leakages, after sponge failure (30,31). However, complexity and cost of stent changes has limited its wide adoption.
There are no reports in the literature of combined EVT and FSEMS in subsequent steps. We have hypothesized that this relay therapy (RT) could reduce time of recovery and number of procedures required to heal an esophageal leak. The main concept of RT would be limiting the number of endoscopies to that needed to get the patient out of the acute phase and using a single FSEMS implantation to allow final chronic healing. We present here our initial experience using RT as a treatment for EAL after minimally invasive esophagectomy. We present the following article in accordance with the STROBE reporting checklist (available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-44/rc).
Methods
We have evaluated patients who developed EAL after undergoing an Ivor Lewis MIE (IL-MIE) with hand-sewn anastomosis at Fundación Favaloro University Hospital and Sanatorio Finchietto in Buenos Aires, Argentina from January 2018 to January 2021. All patients were treated with RT.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Ethics Committee of the Favaloro Fundation University Hospital approved the protocol [approval number: DDI (1301) 1515 CBE 546/15] and informed consent was waived due to the retrospective nature of the study.
Demographic characteristics, pre-operative clinical measurements, perioperative outcomes and postoperative morbidity and mortality of the patients were also recorded on the database (Table 1). All patients were evaluated at the tumor board and neoadjuvant therapy was proposed in patients with T2c tumors or more [TNM for esophagogastric junction tumors and esophageal tumors (32)]. IL-MIE using a gastric tube was offered as the operation of choice to all patients.
Table 1
| Leak patients, N=6 | |
|---|---|
| Age, years | 55.5 (50–57) |
| Gender | |
| Male | 6 (100.0) |
| Female | 0 (0) |
| Type of carcinoma | |
| Squam cell carcinoma | 0 (0) |
| Adeno carcinoma | 6 (100.0) |
| T-stage | |
| T0 | 1 (16.7) |
| T1 | 1 (16.7) |
| T2 | 3 (50.0) |
| T3 | 1 (16.7) |
| N-stage | |
| N0 | 3 (50.0) |
| N1 | 2 (33.3) |
| N2 | 1 (16.7) |
| N3 | 0 (0) |
| R-stage | |
| R0 | 6 (100.0) |
| R1 | 0 (0) |
| Neoadjuvant therapy | |
| Chemotherapy | 2 (33.3) |
| Chemotherapy + radiotherapy | 3 (50.0) |
| No treatment | 1 (16.7) |
| Hospital stay (d) | 16.5 (13–28) |
| Length of ICU-stay (d) | 12 (8–17) |
| Anastomotic stricture | 2 (33.3) |
| Follow-up (m) | 24.53 (16.1–29.8) |
| Mortality rate | 0 (0) |
Values are expressed as median ± IQR. IL-MIE, Ivor Lewis minimally invasive esophagectomy; d, days; m, months; ICU, intensive care unit.
Diagnosis of EAL was suspected by clinical and laboratory signs (33,34) and confirmation was made by either oral and intravenous contrast-enhanced computed tomography and/or endoscopy. EALs were classified as type I (They do not require changes in therapy, medical treatment or diet modification); type II (They require interventional but not surgical treatment.); and type III (require surgical intervention) (2,35). All patients were initially treated with EVT until clinical and laboratory signs of infection were normalized and no worsening condition was observed for at least 48 h. EVT exchanging interval was individually decided for each case depending on clinical and laboratory signs as well as volume of aspiration and endoscopic aspect of the intermediate cavity. After EVT, a FSEMS was deployed to facilitate definite sealing of the leak and to accelerate reinstatement of oral feeding. An algorithm for this relay therapy is proposed in Figure 1.
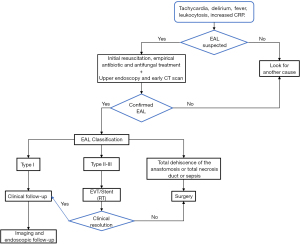
Endpoints included time of EVT start, number of vacuum system changes, time of FSEMS placement, time to final healing, ICU and hospital stay, morbidity and mortality rates, and stricture rate with follow-up at 6 months.
Relay therapy procedure
EVT was placed in the operating room, endoscopic room, or in the ICU under general anesthesia (sedation) or with endotracheal intubation (always safer if high intragastric liquid volumes are suspected). Endoscopic evaluation allowed for identification and characterization of the wall defect and size of intermediate cavity (if any). Subsequently, endoscopic irrigation and debridement was performed to remove collections or contaminated material and sepsis source elimination.
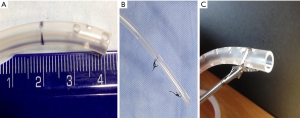
Once the cavity was cleaned, a multifenestrated nasogastric tube (NGT) from 16 to 18 Fr (Figure 2A) with two distal sutures loops [one at the tip and another 3 to 5 cm from the former (Figure 2B)] was introduced into the patient’s nares and advanced to the proximal esophagus. The tube was then inserted in the intermediate cavity making sure to have at least one fenestration every one centimeter and that no fenestrations were left outside the cavity in the esophageal lumen once vacuum was instated. This was tailored made for each patient by measuring the intermediate cavity with the scope and extracting measurements off the CT-Scan when possible. Tubing fenestrations were customized to these measurements. Finally, an endo grasper was used to grab the proximal loop and to hold the tube inside the cavity while vacuum was started (Figure 2C). Vacuum pressure was set within a range of 100–125 mmHg using the building’s central vacuum system at continuous flow until collapse of the cavity was observed endoscopically. NGT was secured to the nose with a 2.0 non-reabsorbable suture.
Endoscopic follow-up was routinely performed at 48 hours to assess improvement, rewashing and repositioning if needed. Flushing of the tubing with 10 to 20 mL of physiological solution was performed every 24 hours. After that, new endoscopies were indicated only if signs of infections were not fully resolved or worsening of any condition was observed.
After all signs of sepsis were resolved and maintained stable for at least 48 h. a new endoscopy was performed to evaluate the aspect of the intermediate cavity. If the cavity appeared covered with healthy granulation tissue but remained large, we proceeded to deploy the FSEMS (Fully covered Wall Flex™ 23 mm × 125 mm Esophageal Stent, Boston Scientific Corp. Natick-Massachusetts, USA).
Briefly, stent placement was performed under direct side-by-side endoscopic visualization. The upper end of the stent was identified through the sheath and positioned 5 cm above the leak. Deployment was made by pulling the covering sheath backwards until the upper end of the stent was fully opened. One week after deployment, soluble contrast swallow was performed to verify stent position and tight sealing of the cavity. If successful, patient was reinstated with oral feeding. Extraction of FSEMS was scheduled 4 weeks after if no unexpected occurrences were found.
Statistical analysis
Descriptive statistics were performed. Categorical variables were expressed as percentages with their corresponding 95% confidence intervals (95% CI). Continuous variables were expressed as means or medians, according to distribution of the variable, with their corresponding standard deviations (SD) or interquartile ranges (IQR). All analyses were conducted using the Statistical Package for Social Sciences software (version 22.0, SPSS Inc., Chicago, IL, USA).
Results
A total of 6 patients were treated with RT for EAL. All patients were male with a median age of 55.5 years (IQR 50–57). Tumor type was adenocarcinoma in all patients. Neoadjuvant therapy was performed in 5 patients (83.3%).
Regarding the type of leak, five patients (83.3%) had type II and one patient (16.7%) had type III anastomotic leak. The leak rate for the series was 14% (6/41). The median time of appearance of the leak was 2 days (IQR 2–2.75). EVT was completed in all patients (6/6) after an average of 7 days (IQR 6.5–7.75), requiring 2 different EVAC changes (IQR 2–2.75). Reoperation was required in one case for anastomotic dehiscence and empyema.
The time of FSEMS placement was 7.1 days (IQR 6–8.25). The median time of defect close was 19.5 days (IQR 17–21.5). The migration rate needing repositioning of FSEMS was 33.3% (2 patients). There were no complications directly related to the use of RT. Imaging (CT with oral contrast, esophagram or upper GI) was used in all patients to confirm complete closure. The median length of ICU and hospital stay was 12 days (IQR 8–17) and 16.5 days (IQR 13–28) respectively. RT characteristics and results are given in Table 2.
Table 2
| Leak patients, N=6 | |
|---|---|
| Type of anastomotic leak | |
| I | 0 (0) |
| II | 5 (83.3) |
| III | 1 (16.7) |
| Time of appearance (d) | 2 (2–2.75) |
| EVT therapy completed | 6 (100.0) |
| Time of EVT therapy (d) | 7 (6.5–7.75) |
| EVT system changes | 2 (2–2.75) |
| Time of FSEMS placement | 7.1 (6–8.25) |
| Need for reoperation | 1 (16.7) |
| Time of defect close (d) | 19.5 (17–21.5) |
Values are expressed as median ± IQR. EVT, endoscopic vacuum therapy; FSEMS, fully covered self-expanding metal stents.
No deaths occurred in the 30 days following RT. Two strictures occurred in the follow-up at 6 months (33.3%). These patients started experiencing mild dysphagia four months after surgery that turned out in anastomotic strictures and required endoscopic dilatation (rule of three sessions every three weeks was performed).
A leak resolution case with RT from one of the study patients is depicted in Figures 3,4.
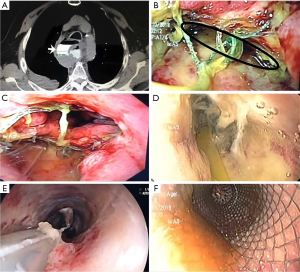
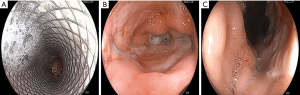
Discussion
In this study, we describe our initial experience with RT for EAL after IL-MIE in patients treated for esophageal cancer. We have found that RT was feasible, safe, effective and reproducible with a success rate comparable to other endoscopic therapies for EAL treatment.
With the stent placement and the advent of EVT, conservative management of these patients has increased with only a few of them requiring surgical intervention (31). This has also been the case in our series where six patients with anastomotic leaks were effectively treated using RT with low need of reoperation. This is the first evidence reported for an RT approach that combines EVT and FSEMS. The high success rate for healing EAL has the potential to reduce recovery time and the number of endoscopic procedures to heal an esophageal leak, resuming oral feeding early after stent placement.
EVT is a relatively new technique so no standardized indications have been established yet. Particularly for the management of leaks in IL-MIE, all patients with acute or chronic anastomotic defects are candidates for EVT. Early detection has the potential to reduce morbidity and mortality. Larger defects typically associated with fluid collections, are the clearest indication for EVT, and studies have shown high efficacy rates of healing associated with this technique (26). Min et al. (36) analyzed 20 patients who were successfully treated with EVT in 95% of cases. The median duration of this treatment was 14.5 days with a median of 5 system changes, and the median admission duration was 49 days. Schorsch et al. (22) and Laukoetter et al. (37) reported success in 20 of 21 (95.2%) and 36 of 39 (92.3%) cases of post-esophagectomy or gastrectomy anastomotic leak, respectively. Kuehn et al. (38) found a successful with EVT for upper gastrointestinal defects in 19 of 21 patients (90.5%). The vacuum system is typically exchanged every few days for continued drainage until closure of the defect, with studies showing an average of 5–6 device changes per patient (3,36,37,39,40).
The need for multiple changes in the system with serial endoscopic controls must be taken into consideration as it represents a burden for the patient and the health care system. Furthermore, using EVT as individual therapy increases the days of treatment increasing the risk of complications due to the use of negative pressure (prolonged use of the sponge and vacuum therapy has associated complications of sponge dislocation and potential erosion into neighboring vital structures). In the quest to improve these limitations, we associated this promising therapy with FSEMS in relay fashion, which has the potential to reduce the number of system changes, require fewer days of EVT, shorter ICU admission and shorter total length of stay.
The use of stents for the primary treatment of leaks and the closure of fistulas by EAL has been compared with EVT, showing a lower success rate (10). In addition, a known pitfall of endoluminal stent placement is the high rate of stent migration (16–62%). In addition to that, complications that arise during stent placement and removal, such as tissue overgrowth and erosion when left in place for longer periods of time have decreased enthusiasm for its use in complex leaks (31,41). The success of an esophageal stent depends on the size of the defect and control of the source, which often requires concomitant drainage and antibiotics.
An attempt to solve some of the above mentioned limitations is the Stent-over-sponge or SOS therapy (29,30). This approach combines EVT and FSEMS placed at the same time. The two major advantages of SOS over EVT alone are: the SEMS helps to direct the vacuum force towards the defect cavity by sealing the endosponge towards the gastrointestinal lumen and this results in faster and more efficient cleansing and ultimately closure of the defect; and the patent esophageal lumen, ensured by the SEMS, allows for oral fluid and food intake, or even placement of a feeding tube. The limitation of this approach seems to be the impossibility of washing the cavity in large defects and the difficulty in changing the vacuum system if necessary.
Our initial experience with RT showed that it has the potential benefits of EVAC and SEMS overcoming most of its limitations. EVAC changes are not limited by the stent, and number of EVAC changes seem to be reduced compared to existing literature. Stents are placed once thick granulation tissue is in place therefore reducing the chances of erosion to neighboring structures.
In summary, RT is a promising new approach for upper gastrointestinal leaks and perforations management. EVT and FSEMS were used separately or combined, but there is no evidence in the literature of their use in relay fashion. In this short series of patients, we have shown that RT poses the potential to overcome several limitations of previous procedures. Larger studies are needed to make a standardized indication for these patients.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Esophagus for the series “Anastomotic Techniques for Minimally Invasive Esophagectomy and Endoscopic Handling of Its Complications”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-44/rc
Data Sharing Statement: Available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-44/dss
Peer Review File: Available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-44/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-44/coif). The series “Anastomotic Techniques for Minimally Invasive Esophagectomy and Endoscopic Handling of Its Complications” was commissioned by the editorial office without any funding or sponsorship. AN served as an unpaid Guest Editor of the series and serves as an unpaid editorial board member of Annals of Esophagus from February 2020 to January 2022. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Ethics Committee of the Favaloro Fundation University Hospital approved the protocol [approval number: DDI (1301) 1515 CBE 546/15] and informed consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Flanagan JC, Batz R, Saboo SS, et al. Esophagectomy and Gastric Pull-through Procedures: Surgical Techniques, Imaging Features, and Potential Complications. Radiographics 2016;36:107-21. [Crossref] [PubMed]
- Low DE, Alderson D, Cecconello I, et al. International Consensus on Standardization of Data Collection for Complications Associated With Esophagectomy: Esophagectomy Complications Consensus Group (ECCG). Ann Surg 2015;262:286-94. [Crossref] [PubMed]
- Famiglietti A, Lazar JF, Henderson H, et al. Management of anastomotic leaks after esophagectomy and gastric pull-up. J Thorac Dis 2020;12:1022-30. [Crossref] [PubMed]
- Fumagalli U, Baiocchi GL, Celotti A, et al. Incidence and treatment of mediastinal leakage after esophagectomy: Insights from the multicenter study on mediastinal leaks. World J Gastroenterol 2019;25:356-66. [Crossref] [PubMed]
- Van Daele E, Van de Putte D, Ceelen W, et al. Risk factors and consequences of anastomotic leakage after Ivor Lewis oesophagectomy†. Interact Cardiovasc Thorac Surg 2016;22:32-7. [Crossref] [PubMed]
- Schröder W, Raptis DA, Schmidt HM, et al. Anastomotic Techniques and Associated Morbidity in Total Minimally Invasive Transthoracic Esophagectomy: Results From the EsoBenchmark Database. Ann Surg 2019;270:820-6. [Crossref] [PubMed]
- Messager M, Warlaumont M, Renaud F, et al. Recent improvements in the management of esophageal anastomotic leak after surgery for cancer. Eur J Surg Oncol 2017;43:258-69. [Crossref] [PubMed]
- Lee DK, Min YW. Role of Endoscopic Vacuum Therapy as a Treatment for Anastomosis Leak after Esophageal Cancer Surgery. Korean J Thorac Cardiovasc Surg 2020;53:205-10. [Crossref] [PubMed]
- Persson S, Elbe P, Rouvelas I, et al. Predictors for failure of stent treatment for benign esophageal perforations - a single center 10-year experience. World J Gastroenterol 2014;20:10613-9. [Crossref] [PubMed]
- Rausa E, Asti E, Aiolfi A, et al. Comparison of endoscopic vacuum therapy versus endoscopic stenting for esophageal leaks: systematic review and meta-analysis. Dis Esophagus 2018; [Crossref] [PubMed]
- Hünerbein M, Stroszczynski C, Moesta KT, et al. Treatment of thoracic anastomotic leaks after esophagectomy with self-expanding plastic stents. Ann Surg 2004;240:801-7. [Crossref] [PubMed]
- Doniec JM, Schniewind B, Kahlke V, et al. Therapy of anastomotic leaks by means of covered self-expanding metallic stents after esophagogastrectomy. Endoscopy 2003;35:652-8. [Crossref] [PubMed]
- Gelbmann CM, Ratiu NL, Rath HC, et al. Use of self-expandable plastic stents for the treatment of esophageal perforations and symptomatic anastomotic leaks. Endoscopy 2004;36:695-9. [Crossref] [PubMed]
- Kauer WK, Stein HJ, Dittler HJ, et al. Stent implantation as a treatment option in patients with thoracic anastomotic leaks after esophagectomy. Surg Endosc 2008;22:50-3. [Crossref] [PubMed]
- Langer FB, Wenzl E, Prager G, et al. Management of postoperative esophageal leaks with the Polyflex self-expanding covered plastic stent. Ann Thorac Surg 2005;79:398-403; discussion 404. [Crossref] [PubMed]
- Lee BI, Choi KY, Kang HJ, et al. Sealing an extensive anastomotic leak after esophagojejunostomy with an antimigration-modified covered self-expanding metal stent. Gastrointest Endosc 2006;64:1024-6. [Crossref] [PubMed]
- Peters JH, Craanen ME, van der Peet DL, et al. Self-expanding metal stents for the treatment of intrathoracic esophageal anastomotic leaks following esophagectomy. Am J Gastroenterol 2006;101:1393-5. [Crossref] [PubMed]
- Smallwood NR, Fleshman JW, Leeds SG, et al. The use of endoluminal vacuum (E-Vac) therapy in the management of upper gastrointestinal leaks and perforations. Surg Endosc 2016;30:2473-80. [Crossref] [PubMed]
- Ahrens M, Schulte T, Egberts J, et al. Drainage of esophageal leakage using endoscopic vacuum therapy: a prospective pilot study. Endoscopy 2010;42:693-8. [Crossref] [PubMed]
- Brangewitz M, Voigtländer T, Helfritz FA, et al. Endoscopic closure of esophageal intrathoracic leaks: stent versus endoscopic vacuum-assisted closure, a retrospective analysis. Endoscopy 2013;45:433-8. [Crossref] [PubMed]
- Schniewind B, Schafmayer C, Voehrs G, et al. Endoscopic endoluminal vacuum therapy is superior to other regimens in managing anastomotic leakage after esophagectomy: a comparative retrospective study. Surg Endosc 2013;27:3883-90. [Crossref] [PubMed]
- Schorsch T, Müller C, Loske G. Endoscopic vacuum therapy of perforations and anastomotic insufficiency of the esophagus. Chirurg 2014;85:1081-93. [Crossref] [PubMed]
- Bludau M, Hölscher AH, Herbold T, et al. Management of upper intestinal leaks using an endoscopic vacuum-assisted closure system (E-VAC). Surg Endosc 2014;28:896-901. [Crossref] [PubMed]
- Heits N, Stapel L, Reichert B, et al. Endoscopic endoluminal vacuum therapy in esophageal perforation. Ann Thorac Surg 2014;97:1029-35. [Crossref] [PubMed]
- Goenka MK, Goenka U. Endotherapy of leaks and fistula. World J Gastrointest Endosc 2015;7:702-13. [Crossref] [PubMed]
- de Moura DTH, de Moura BFBH, Manfredi MA, et al. Role of endoscopic vacuum therapy in the management of gastrointestinal transmural defects. World J Gastrointest Endosc 2019;11:329-44. [Crossref] [PubMed]
- Weidenhagen R, Hartl WH, Gruetzner KU, et al. Anastomotic leakage after esophageal resection: new treatment options by endoluminal vacuum therapy. Ann Thorac Surg 2010;90:1674-81. [Crossref] [PubMed]
- Wedemeyer J, Brangewitz M, Kubicka S, et al. Management of major postsurgical gastroesophageal intrathoracic leaks with an endoscopic vacuum-assisted closure system. Gastrointest Endosc 2010;71:382-6. [Crossref] [PubMed]
- Gubler C, Schneider PM, Bauerfeind P. Complex anastomotic leaks following esophageal resections: the new stent over sponge (SOS) approach. Dis Esophagus 2013;26:598-602. [Crossref] [PubMed]
- Valli PV, Mertens JC, Kröger A, et al. Stent-over-sponge (SOS): a novel technique complementing endosponge therapy for foregut leaks and perforations. Endoscopy 2018;50:148-53. [Crossref] [PubMed]
- Fabbi M, Hagens ERC, van Berge Henegouwen MI, et al. Anastomotic leakage after esophagectomy for esophageal cancer: definitions, diagnostics, and treatment. Dis Esophagus 2021;34:doaa039.
- Brierley J, Gospodarowicz MK, Wittekind CH, editors. TNM classification of malignant tumours. 8th edition. Oxford, UK; Hoboken, NJ: John Wiley & Sons, Inc.; 2017.
- Straatman J, Harmsen AM, Cuesta MA, et al. Predictive Value of C-Reactive Protein for Major Complications after Major Abdominal Surgery: A Systematic Review and Pooled-Analysis. PLoS One 2015;10:e0132995. [Crossref] [PubMed]
- Park JK, Kim JJ, Moon SW. C-reactive protein for the early prediction of anastomotic leak after esophagectomy in both neoadjuvant and non-neoadjuvant therapy case: a propensity score matching analysis. J Thorac Dis 2017;9:3693-702. [Crossref] [PubMed]
- Dent B, Griffin SM, Jones R, et al. Immanuel and N. Hayes. Management and outcomes of anastomotic leaks after Oesophagectomy. Br J Surg 2014;101:6.
- Min YW, Kim T, Lee H, et al. Endoscopic vacuum therapy for postoperative esophageal leak. BMC Surg 2019;19:37. [Crossref] [PubMed]
- Laukoetter MG, Mennigen R, Neumann PA, et al. Successful closure of defects in the upper gastrointestinal tract by endoscopic vacuum therapy (EVT): a prospective cohort study. Surg Endosc 2017;31:2687-96. [Crossref] [PubMed]
- Kuehn F, Schiffmann L, Janisch F, et al. Surgical Endoscopic Vacuum Therapy for Defects of the Upper Gastrointestinal Tract. J Gastrointest Surg 2016;20:237-43. [Crossref] [PubMed]
- Wedemeyer J, Schneider A, Manns MP, et al. Endoscopic vacuum-assisted closure of upper intestinal anastomotic leaks. Gastrointest Endosc 2008;67:708-11. [Crossref] [PubMed]
- Mennigen R, Senninger N, Laukoetter MG. Endoscopic vacuum therapy of esophageal anastomotic leakage. Gastrointest Endosc 2015;82:397. [Crossref] [PubMed]
- Dasari BV, Neely D, Kennedy A, et al. The role of esophageal stents in the management of esophageal anastomotic leaks and benign esophageal perforations. Ann Surg 2014;259:852-60. [Crossref] [PubMed]
Cite this article as: Turchi MJ, Llanos FL, Ramirez MG, Badaloni F, Nachman F, Nieponice A. Relay therapy with endovac and endoscopic stents for anastomotic leaks after minimally invasive esophagectomy. Ann Esophagus 2022;5:20.


