
Multimodal therapy for esophageal squamous cell carcinoma according to TNM staging in Japan—a narrative review of clinical trials conducted by Japan Clinical Oncology Group
Introduction
It is well-recognized around the world that multimodal therapeutic modalities have led to improved oncologic outcomes in patients with esophageal squamous cell carcinoma (ESCC) (1). In Japan, transthoracic esophagectomy combined with cervical, thoracic, and abdominal (three-field) lymphadenectomy for advanced thoracic ESCC is developed and adopted as a standard surgical procedure (2). In particular, we do not consider supraclavicular lymph node (LN) metastasis as distant metastasis, and have been actively performing surgery for resectable cancer. Therefore, multimodal treatment for ESCC is used to improve the surgical outcomes after relatively invasive surgery in Japan, whereas in the West, multimodal treatment is employed after Ivor-Louis esophagectomy. In addition to the differences in the surgical techniques adopted, multimodal treatment strategies for advanced ESCC have also developed in a different way between Japan and Western countries.
In consideration of these unique features of surgery in Japan, the Japan Esophageal Oncology Group (JEOG), which is a subgroup of the Japan Clinical Oncology Group (JCOG), has established an optimized treatment strategy for ESCC based on the results of multicenter trials (3). Herein, we discuss the historical and current state of treatment strategies for ESCC in Japan according to the state of progression of ESCC, focusing on the results of JEOG’s clinical studies and the contents of the Japanese Guidelines for the treatment of ESCC. The stage is described according to the Union for International Cancer Control (UICC), but it should be noted that supraclavicular LN metastasis is considered resectable, as mentioned above. We present the following article in accordance with the Narrative Review reporting checklist (available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-22/rc).
Therapy for cT1aN0M0 ESCC
Endoscopic resection (ER), including endoscopic mucosal resection (EMR) and endoscopic submucosal resection (ESR), is primarily performed for superficial esophageal carcinoma confined to the mucosa. Technical advances in ESR have made it possible for early ESCC to be resected in an en bloc manner, irrespective of the tumor size. Indication for ER is mainly determined by the risk of LN metastasis. For superficial cancers invading mucosal epithelium (EP) or lamina propria mucosae (LPM) (T1a-EP or LPM) without lymphovascular invasion, curative ER can be expected, because the risk of LN metastasis is thought to be less than 5% (4,5). Thus, T1a-EP and LPM are accepted as absolute indications for ER. Meanwhile, superficial cancer invading the muscularis mucosae (MM) or superficial submucosa up to 200 µm (SM1) is reported to be associated with an approximately 20% risk of LN metastasis (5,6). Therefore, if histopathological examination of the resected specimen shows a greater depth of invasion than the MM or lymphovascular invasion, additional treatment should be considered because of the increased risk of LN metastasis. Esophagectomy remains highly invasive, and it has been debated as to whether less invasive modalities could be applied to cases of endoscopic non-curative resection with a lower risk of recurrence as compared to stage II or more advanced cancer. Therefore, the JCOG carried out a phase II study (JCOG0508, 2006-2012) to confirm the efficacy and safety of chemoradiotherapy (CRT) for cases of superficial ESCC with non-curative ER (Table 1). Based on the histopathological findings after ER, patients received the following treatments: no additional treatment for patients with pT1a tumors with a negative resection margin and no lymphovascular invasion (group A); prophylactic CRT at a radiation dose of 41.4 Gy delivered to locoregional LNs for patients with pT1a tumors with lymphovascular invasion or pT1b tumors with a negative resection margin (group B); definitive CRT (dCRT) (50.4 Gy) with a 9-Gy boost to the primary site for patients with a positive vertical resection margin (group C). The 3-year overall survival (OS) rate was 90.7% in group B [90% confidence interval (CI), 84.0–94.7%) and 92.6% in the entire population of patients (90% CI, 88.5–95.2%). The efficacy of the new strategy of selective CRT based on diagnostic ER was comparable to that of surgery, and the combination of ER and selective CRT might be considered as a minimally invasive treatment option (7). Based on these results, not only surgical resection, but also CRT has become the standard of care for patients in whom ER is demonstrated to be non-curative (Figure 1).
Table 1
| Trial | Year | Stage enrollment† | Phase | Arm | n | Primary endpoint | P value | Summary |
|---|---|---|---|---|---|---|---|---|
| JCOG0508 | 2006–2012 | Clinical T1a (after ER) | II | No additional treatment for pT1a | 74 | N.A. | N.S. | 3-year OS of all patients was 92.6%. Efficacy of selective CRT based on diagnostic ER was comparable to that of surgery |
| CRT (41.4 Gy) for pT1a with lymphovascular invasion/pT1b | 87 | 3-year OS: 90.7% | ||||||
| CRT (50.4 Gy) for tumors with a positive margin | 15 | N.A. | ||||||
| JCOG9708 | 1997–2000 | T1bN0M0 | II | CRT (60 Gy) | 72 | 5-year OS: 75.5% | CRT could be considered as a candidate of promising treatment to be compared with surgery in patients with Stage I ESCC | |
| CR rate: 87.5% | ||||||||
| JCOG0502 | 2006–2013 | T1bN0M0 | parallel-group controlled trial | Surgery | 209 | 5-year OS: 86.5% | N.S. | CRT was considered as a treatment option for stage I ESCC with organ preservation |
| CRT (60 Gy) | 159 | 5-year OS: 85.5% |
†, UICC at the time. ESCC, esophageal squamous cell carcinoma; ER, endoscopic resection; CRT, chemoradiotherapy; OS, overall survival; N.A., not available; N.S., not significant; CR, complete response.
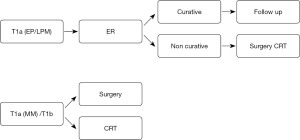
Therapy for cT1bN0M0 ESCC
The standard treatment for stage I ESCC that is not amenable to endoscopic therapy is surgery, but as mentioned above, the high invasiveness of the surgery is a considerable problem. The JCOG conducted a phase II study (JCOG9708, 1997-2000) to confirm the efficacy and safety of CRT for cT1bN0 ESCC. Treatment consisted of cisplatin 70 mg/m2 (day 1) and 5-FU 700 mg/m2/day (days 1–4) combined with 30 Gy radiotherapy (2 Gy/day, 5 days/week, days 1–21). The treatment cycle was repeated twice with 1-week interval between the two cycles. Among 72 patients, complete response (CR) was achieved in 63 (87.5%) patients, and the 5-year survival rate was 75.5% (8). Residual disease was noted in 12.5% and recurrent disease in 41%, but curative resection was achieved by salvage ER or surgery in most of these cases. The high CR and high survival rates suggested that this regimen could be considered as a promising treatment to be compared with surgery in patients with stage I ESCC.
Subsequently, the JCOG planned a randomized controlled trial (RCT) to confirm the non-inferiority of CRT to surgery for stage I ESCC, for which ER is not indicated; however, as patient accumulation was difficult due to problems with obtaining consent for participation, a parallel-group controlled trial (JCOG 0502, 2006-2013) was conducted. The patients were allocated to their preferred arm, namely, the surgery (arm C) or CRT (arm D) arm. CRT consisted of cisplatin plus 5-FU, with concurrent radiation at the dose of 60 Gy. The 3- and 5-year OS rates were 94.7% and 86.5% in arm C, and 93.1% and 85.5% in arm D (adjusted HR 1.05; 95% CI: 0.67–1.64 (<1.78)]. Thus, CRT was considered as a treatment option for stage I ESCC with organ preservation (9). Based on the results of the two studies mentioned above, CRT is considered as an alternative standard of care to surgery for stage I ESCC cases, in which ER is not indicated.
Surgery for stage I/II/III/IVA ESCC (excluding T4b disease)
The standard treatment for resectable advanced ESCC is esophagectomy with LN dissection. As mentioned above, in Japan, transthoracic esophagectomy with three-field LN dissection (3FD) is the standard surgical procedure adopted for advanced thoracic ESCC (2).
Approach for esophagectomy:
Until the first thoracoscopic esophagectomy was published by Cuschieri in 1992 (10), only open thoracic and laparotic techniques were used for ESCC. Since then, techniques of thoracoscopic and laparoscopic surgery have been established rapidly and thoracoscopic and laparoscopic surgery is now recognized to be useful as minimally invasive approaches compared to open surgery. Some thoracoscopic and laparoscopic approaches for resection of thoracic esophageal cancer, based on the tumor location, clinical stage, and patient demographics, have been described as minimally invasive esophagectomy (MIE) (11,12). In 1996, Akaishi et al. first reported the thoracoscopic total esophagectomy and thoracic LNs dissection in Japan (13). Following these explorative researchers, MIE has begun to be performed more widely, and its surgical techniques have become more standardized.
We summarized studies comparing the surgical results of open transthoracic esophagectomy (OE) and MIE, and concluded that MIE was not inferior to OE in accuracy of lymphadenectomy. It would be expected to be much less invasive and may also reduce the risk of pulmonary infections (14).
Kernstine et al. reported about robot-assisted MIE (RAMIE) for the first time in 2004 (15). In 2006, they described the initial clinical experience of using RAMIE in conjunction with traditional laparoscopic surgery, showing the technical feasibility and minimal blood loss of the new surgical technique (16).
We can see the surgical field magnified in three-dimensions by using the da Vinci Robotic Systems (Intuitive, Sunnyvale, CA, USA) (17). Robot-assisted surgery has the capacity to accelerate the learning curve of MIE because of its advantages such as articulation of surgical instruments, tremor filtering, the ability to minimize large movements for the surgeon, and good ergonomics, The increased degree of freedom due to the articulation of the surgical instruments might overcome the limitation of movement by the thorax and improve the accuracy of lymphadenectomy around the recurrent laryngeal nerves (RLN) (18), resulting in improved outcomes and prevention of recurrent laryngeal nerve paralysis (RLNP). Due to these superiorities, RAMIE is rapidly gaining popularity in Japan, but some problems remain, such as the expensive da Vinci Robotic Systems and the clinical benefits of RAMIE when comparing it to thoracoscopic procedures.
Three-field lymphadenectomy:
The significance of LNs dissection in esophagectomy has been well-established worldwide. After the first report of success of esophagectomy by Torek et al. in 1913 (19), the efficacy and safety of esophagectomy for ESCC have been endorsed by many investigators, and the extent of lymphadenectomy has been expended. 3FD was first performed by two Japanese physicians. Kajitani performed systematic dissection of the LN around the RLN and developed upper thoracic lymphadenectomy (20). Then, Sannohe reported cervical lymphadenectomy and the rates of metastases in patients who received 3FD (21). After publication of these two reports, a number of reports corroborating the safety and survival-prolonging effect of 3FD have been published in Japan (22-25). Initially, the surgery was performed without attention paid to preserving the RLN, and RLNP occurred in a lot of cases. However, the frequency of RLNP has gradually decreased as the surgical skills around the RLN have improved. 3FD had gained worldwide acceptance and its stability was well-established in the 1990’s (26-28). Kato et al. showed that patients with ESCC who underwent 3FD had a favorable OS rates compared to those who underwent two-field LN dissection (2FD) (29). Igaki et al. reported that cervical lymphadenectomy is also of importance even in patients whose tumor is located in lower thoracic esophagus. They reported that 3FD for patients with LN metastases in the upper and/or central mediastinum can improve the survival rate as compared to 2FD, even in patients with lower thoracic ESCC (2). Furthermore, Altorki et al. described that of 80 patients who underwent esophagectomy with 3FD, 30% showed postoperative upstaging of the disease (26). On the basis of these results, in Japan, esophagectomy combined with 3FD is now the standard surgical strategy for thoracic ESCC.
3FD not only allows resection of a large number of LNs that could be potentially metastatic, but also allows more accurate staging. Moreover, addition of a cervical approach may also improve the accuracy of mediastinal LN dissection, which has been shown to have the greatest prognostic impact.
In recent years, there have been some systematic reviews and meta-analyses published. Shang et al. performed an analysis of the long-term survival of the ESCC patients and found that 3FD was preferable to 2FD in patients with cervical or upper mediastinal LNs metastasis (30). Ma et al. also performed a meta-analysis and described that 3FD was related to improved survival after esophagectomy (31).
Adjuvant therapy for stage I/II/III/IVA ESCC (excluding T4b disease)
Preoperative and postoperative radiotherapy
In the 1970’s, preoperative radiotherapy was the main preoperative treatment for ESCC. It was widely believed to increase the possibility of resection of the primary lesion and to avoid local tumor relapse (32). In contrast, the advantage of postoperative radiation therapy was highlighted by several researchers, who described reduced postoperative morbidity as well as improved survival according to a retrospective comparing with a control group (33). Hence, the JEOG carried out a RCT (JCOG 8201, 1981–1983) to determine whether it is preoperative or postoperative radiotherapy that might yield better survival rates (Table 2). This study contrasted preoperative radiation therapy (30 Gy) plus postoperative radiation therapy (24 Gy) with only postoperative radiation therapy (50 Gy). The outcome showed that the group with surgery plus postoperative radiation therapy alone had a significantly higher survival rate than the group with surgery plus preoperative radiation therapy plus postoperative radiation therapy (34). Based on these results, the timing of adjuvant radiation therapy for ESCC was changed from preoperative to postoperative.
Table 2
| Trial | Year | Stage enrollment† | Phase | Arm | n | Primary endpoint | P value | Summary |
|---|---|---|---|---|---|---|---|---|
| JCOG8201 | 1981–1983 | I–III | III | Preoperative + postoperative RT | 104 | OS‡: 13.1 | <0.01 | Postoperative RT alone group was superior |
| Postoperative RT alone | 103 | OS‡: 21.6 | ||||||
| JCOG8503 | 1984–1987 | I–IV (resectable) | III | Postoperative RT | 127 | 5-year OS: 44% | N.S. | |
| Postoperative CT (CV) | 126 | 5-year OS: 42% | ||||||
| JCOG8806 | 1988–1991 | I–IV (resectable) | III | Surgery alone | 100 | 5-year OS: 44.9% | N.S. | |
| Surgery + postoperative CT (CV) | 105 | 5-year OS: 48.1% | ||||||
| JCOG9204 | 1992–1997 | II/III, excluding T4 | III | Surgery alone | 122 | 5-year DFS: 45% | 0.04 | Surgery + postoperative CT arm was advantage |
| Surgery + postoperative CT (CF) | 120 | 5-year DFS: 55% | ||||||
| JCOG9907 | 2000–2006 | II/III, excluding T4 | III | Preoperative CT (CF) | 164 | 5-year OS: 55% | 0.04 | Preoperative CT group was advantage |
| Postoperative CT (CF) | 161 | 5-year OS: 43% | ||||||
| JCOG1109 | 2012–2018 | II/III, excluding T4 | III | Preoperative CT (CF) | OS | Follow-up is ongoing | ||
| Preoperative CT (DCF) | ||||||||
| Preoperative CRT |
†, UICC at that time; ‡, median, month. ESCC, esophageal squamous cell carcinoma; RT, radiation therapy; OS, overall survival; CT, chemotherapy; CV, cisplatin plus vindesine; N.S., not significant; DFS, disease-free survival; CF, cisplatin plus 5-fluorouracil; DCF, docetaxel, cisplatin plus 5- fluorouracil; CRT, chemoradiotherapy.
Postoperative radiation therapy versus postoperative chemotherapy
Cisplatin has been recognized as a valuable agent for the therapy of ESCC since early 1980’s in Japan. The JEOG carried out a RCT (JCOG8503, 1984–1987) to investigate whether postoperative radiotherapy or postoperative chemotherapy was associated with better survival. This study was designed to compare postoperative radiation therapy (50 Gy) with postoperative chemotherapy (2 courses of cisplatin 70 mg/m2 + vindesine 3 mg/m2). At that time, 5-FU had not yet become widespread, and the combination therapy of cisplatin and vindesine, which was the standard of care for non-small cell lung carcinoma at that time, was employed. Though the trial did not show any significant difference in the 5-year OS rate between the two treatment groups, it at least indicated that postoperative chemotherapy was not less effective than postoperative radiation therapy, which was the worldwide standard treatment at that time (35). Therefore, cisplatin-based chemotherapy became a commonly acceptable postoperative therapy for ESCC in Japan.
Postoperative chemotherapy versus surgery alone
Surgical techniques used in the treatment of ESCC have made remarkable progress, especially the development of a technique that specifically resect the superior thoracic and cervical LN, which have become standard practice in Japan since the later 1980’s. Thus, the JEOG carried out a RCT (JCOG 8806, 1988–1991) to determine whether postoperative chemotherapy would increase the survival rate of patients who underwent esophagectomy combined with 3FD. The trial compared surgery alone with surgery plus postoperative chemotherapy (2 courses of cisplatin 70 mg/m2 + vindesine 3 mg/m2) and found no significantly different 5-year OS rates in the two arms, so the esophagectomy and 3FD without adjuvant therapy were accepted as the standard treatment for ESCC at the time (36).
Subsequently, in two phase II studies, CF was shown to be preferable to cisplatin plus vindesine as postoperative chemotherapy. Thus, the JEOG carried out a RCT (JCOG 9204, 1992–1997) to determine whether postoperative chemotherapy with CF might provide an additive effect on survival compared with surgery plus 2FD or 3FD alone for pathologic stage II or III ESCC (excluding T4 disease). The study evaluated surgery alone versus surgery and postoperative chemotherapy (2 courses of cisplatin 80 mg/m2 on day 1 + 5-FU 800 mg/m2 on days 1–5). Comparing the primary endpoint of 5-year disease-free survival (DFS) rate, the study showed that the postoperative chemotherapy arm (120 patients) had a more favorable DFS rate than the surgery alone arm (122 patients) (55% vs. 45%, P=0.04), and the 5-year OS rates were respectively 61% and 52% (P=0.13). In the LN metastatic subgroup, the benefits in outcomes gained with surgery plus postoperative treatment were more marked (37). In accordance with these results, in the latter half of the 1990’s, surgery with subsequent postoperative CF therapy was regarded as the standard treatment for advanced ESCC.
Postoperative chemotherapy versus preoperative chemotherapy
Whereas postoperative chemotherapy was the standard treatment for ESCC in Japan, preoperative chemotherapy was the predominant therapy in the Western countries because of the surgical aggressiveness and high rate of complication (38). This led to a debate on whether preoperative chemotherapy was more effective in increasing the survival rate of patients of ESCC in comparison to surgery alone or surgery and subsequent chemotherapy. Thus, the JEOG carried out a RCT (JCOG9907, 2000–2006) to investigate the best timing (preoperative vs. postoperative) of chemotherapy in locally advanced ESCC patients. In the trial, clinical stage II/III ESCC patients, except T4 patients, were randomized to receive preoperative or postoperative chemotherapy (2 courses of cisplatin 80 mg/m2 on day 1 and continuous infusion of 5-FU 800 mg/m2 on days 1–5, with a 3-week interval). The primary endpoint was progression-free survival (PFS), which did not touch the stopping boundary. However, OS was more favorable in the preoperative chemotherapy arm (164 patients) than in the postoperative chemotherapy arm (166 patients) (P=0.01). In the latest analysis, the 5-year OS rate of the postoperative chemotherapy arm was 43% compared with 55% in the preoperative chemotherapy arm [hazard ratio (HR), 0.73, 95% CI: 0.54–0.99, P=0.04] (39). Moreover, preoperative chemotherapy was not related to an increased risk of postoperative morbidity and mortality in the hospital (40).
It was considered that the downstaging of the disease achieved by preoperative chemotherapy in some patients may have contributed to the improved prognosis in the preoperative chemotherapy group. The clinical stage after randomization did not differ between the two groups, whereas the pathological stage was significantly lower in the preoperative chemotherapy group than in the postoperative chemotherapy group. In addition, a higher frequency of complete resection (R0) and a higher rate of completion of the protocol treatment may also have contributed to the improved prognosis in the preoperative chemotherapy group. In the preoperative chemotherapy arm, 85.4% of patients successfully finished the protocol treatment consisting of two courses of chemotherapy and complete resection, compared to only 75.0% of the patients in postoperative chemotherapy arm. Based on the results, preoperative chemotherapy using CF has been standardized as a treatment for stage II/III ESCC patients in Japan (Figure 2). Therefore, the best timing for adjuvant chemotherapy again shifted from postoperative to preoperative.
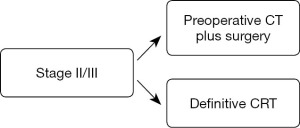
Adequate preoperative therapy
A subgroup analysis was performed in the JCOG9907 trial, which demonstrated that ESCC patients with clinical stage II or T1–2 disease, relatively early-stage disease, were more likely to benefit from preoperative chemotherapy than those with clinical stage III or T3 disease. In addition, the single-site recurrence rates of all recurrence in both arms were found to be low (31% and 25% respectively), which may be caused to precise surgical skills in Japan. The findings of the trial indicated that preoperative chemotherapy using cisplatin and 5-FU may be a suitable treatment option for patients who have achieved sufficient local disease control by aggressive operation, while more intensive adjuvant therapy, such as more potent preoperative chemotherapy or preoperative chemoradiation therapy, may be beneficial for patients with inadequate local disease control.
The need for more aggressive chemotherapy led to the focus on docetaxel which was one of the most promising drugs for the chemotherapy of unresectable ESCC. An exploratory trial of preoperative chemotherapy with docetaxel, cisplatin and 5-FU (DCF) for patients with locally advanced ESCC indicated a good response rate (61.5%), and no therapy-related deaths. The treatment promise of DCF was also proven in a phase II randomized study (41). The clinical issue of which was superior, preoperative chemotherapy or preoperative CRT, however, remained unanswered.
In 2012, the JEOG launched a three-arm RCT (JCOG1109) to evaluate the OS advantage of DCF and chemoradiation therapy with CF (CF-RT) over CF alone for the preoperative treatment of locally advanced ESCC (42). Arm A received preoperative CF (2 courses of cisplatin 80 mg/m2 on day 1, 5-FU 800 mg/m2 on days 1–5, repeated every 3 weeks); Arm B received preoperative DCF (3 courses of docetaxel 70 mg/m2 on day 1, cisplatin 70 mg/m2 on day 1, 5-FU 750 mg/m2 on days 1–5, repeated every 3 weeks); Arm C received preoperative chemoradiation (41.4 Gy/23 fractions) with CF (2 courses of cisplatin 75 mg/m2 on day 1 and 5-FU 1,000 mg/m2 on days 1–4, repeated every 4 weeks). Transthoracic OE and MIE were allowed in all arms, and esophagectomy was required to be scheduled within 56 days after finishing of the preoperative therapy. Registration of patients was accomplished in 2018, and registered patients are still being followed.
Preoperative therapy with immune checkpoint inhibitors
Immune checkpoint inhibitors are a new class of agents with antitumor activity, represented by nivolumab and pembrolizumab. Until lately, any molecular-targeted drugs were not accepted for the therapy of advanced ESCC; however, the FDA accepted pembrolizumab as a second- or subsequent-line therapy for PD-L1-positive patients, in 2019 (43). ESCC typically shows strong PD-L1 expression. Expression in the cancer cells was reported to range from 15% to 83%, and in immunocytes from 13% to 31% (43-46). Additionally, an international phase III study (ATTRACTION-3) demonstrated that nivolumab prolonged OS significantly compared to established taxens in patients with unresectable advanced or recurrent ESCC resistant or intolerant to 5-FU and platinum-based drugs in 2019 (47). Thus, in 2020, nivolumab was accepted as a second-line chemotherapy for patients with unresectable advanced or recurrent ESCC in Japan. Given the results, the JEOG launched a phase I trial (JCOG1804E) to investigate the benefit of preoperative chemotherapy with the combination of CF and DCF plus nivolumab (48).
CRT for stage I/II/III/IVA ESCC (excluding T4b disease)
A phase III study in 1999 in the United States in patients with T1-3N0-1M0 ESCC demonstrated that CRT combined with 50.4 Gy of radiation and CF results in significant improvement in 5-year OS of 26%, compared to 0% with radiotherapy alone (49). These results have made CRT a standard less invasive therapy for patients who do not prefer surgery. Therefore, the JEOG carried out a phase II study (JCOG9906, 2000–2002) to investigate the efficacy and safety of CRT in stage II or III ESCC patients (Table 3). 96 patients underwent CRT, and the results in the CRT group were equivalent to those in the preoperative chemotherapy plus surgery group, with controllable toxicity. Nevertheless, late toxicities, such as Grade 3 or 4 esophagitis (13%), pericardial (16%) and pleural (9%) exudates, and radiation pneumonitis (4%), were noted, resulting in 4 deaths, and it was concluded that further improvement is needed to reduce the rate of late-phase toxicities (50).
Table 3
| Trial | Year | Stage enrollment† | Phase | Arm | n | Primary endpoint | P value | Summary |
|---|---|---|---|---|---|---|---|---|
| JCOG9906 | 2000–2002 | II/III, excluding T4 | II | CRT | 76 | OS‡: 29 | CRT was effective | |
| JCOG0909 | 2010–2014 | II/III, excluding T4 | II | CRT with/without salvage | 94 | 3-year OS: 74.2% | CRT with salvage was effective and safe | |
| JCOG0303 | 2004–2009 | T4b or unresectable LN | II | CRT (standard-dose CF) | 71 | OS‡: 14.4 | N.S. | Low-dose group was slightly inferior |
| CRT (low-dose CF) | 71 | OS‡: 13.1 | ||||||
| COSMOS | 2013–2014 | T4b or unresectable LN | II | indDCF and CS/CRT | 48 | 1-year OS: 67.9% | IndDCF followed by CS showed tolerability and efficacy | |
| JCOG1510 | 2016 | T4b or unresectable LN | III | CRT | OS | Patient enrollment is ongoing | ||
| indDCF and CS/CRT |
†, UICC at that time; ‡, median, month. CRT, chemoradiotherapy; ESCC, esophageal squamous cell carcinoma; OS, overall survival; CF, cisplatin plus 5-fluorouracil; LN, lymph node; N.S., not significant; indDCF, induction therapy with docetaxel, cisplatin, and 5-fluorouracil; CS, conversion surgery.
The combination of dCRT with a radiation dose of 60 Gy with CF therapy is applied to stage II or III ESCC patients who do not prefer surgery in Japan. Due to the high rate of late-phase toxicities and the complications associated with salvage surgery, dCRT for the patients in stage II or III ESCC is one of the alternatives to be under consideration. Therefore, the JEOG carried out a single-arm confirmatory study (JCOG 0909, 2010–2014) to investigate the use of a decreased radiation dose of 50.4 Gy to lower the rate of late-phase toxicities, the incorporation of salvage therapy to improve results, and to evaluate the safety of salvage therapy. The OS rate at 3 years was 74.2% (90% CI, 65.9–80.8%), which was better than the predicted 55%. In salvage surgery, Grade 3 or 4 postoperative complication was observed in 5 patients (20%) and postoperative mortality in 1 patient (4%), but complete resection was achievable in 76% of patients (51). Salvage surgery was regarded as an effective therapy for specific cases, and CRT with 50.4 Gy radiotherapy and CF, became the standard treatment in Japan for ESCC patients who wanted nonsurgical therapy.
CRT for stage IVA(T4b) ESCC
The JEOG carried out a phase II/III trial (JCOG0303, 2004–2009) of CRT with standard-dose CF (Group A) and low-dose CF (Group B) in ESCC patients with T4b disease or unresectable LNs. Since there was no difference in the toxicity between the two group, Group B was not deemed worthy of further investigation in the phase III trial and the trial was discontinued (52). Everyday RT and low-dose CF chemotherapy was not eligible for further assessment as a new therapeutic strategy for locally advanced unresectable ESCC patients.
A multicenter phase II trial (COSMOS trial) was conducted to evaluate the safety and efficacy of induction DCF chemotherapy followed by conversion surgery for initially unresectable locally advanced ESCC in 2013. Conversion surgery was applied to 41.7% of patients following induction chemotherapy or subsequent CRT, and complete resection was successfully performed in 95% of the patients, with no severe postoperative morbidities. Induction DCF chemotherapy and subsequent conversion surgery is a promising multimodal therapeutic strategy for locally advanced unresectable ESCC patients in terms of both the tolerance and efficacy (53). According to the results, the JEOG planned a phase III trial (JCOG1510) to clarify the results of conversion surgery following induction chemotherapy (54). The objective of the trial is to verify the advantage of DCF-based induction chemotherapy followed by conversion surgery or dCRT over dCRT alone in terms of OS in locally advanced unresectable ESCC patients, and patients are currently being enrolled (Figure 3).
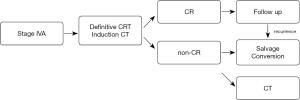
Chemotherapy for stage IVB ESCC
As is the practice worldwide, systemic chemotherapy is used in Japan for cases of unresectable advanced ESCC, with CF therapy being the mainstay. Under this circumstance, the JEOG initiated a RCT (JCOG1314), in 2014, to verify the advantage of DCF over CF as chemotherapy for unresectable advanced or recurrent ESCC in terms of the OS. (55). Patients in group A were treated with CF (cisplatin 80 mg/m2 on day 1, 5-FU 800 mg/m2 on days 1–5, repeated every 4 weeks) and group B were treated with DCF (30 mg/m2 docetaxel on day 1 and 15, 80 mg/m2 cisplatin on day 1, 800 mg/m2 5-FU on days 1–5, repeated every 4 weeks). Patient enrollment is ongoing.
As second-line therapy, taxanes were used, but as mentioned above (see the section of Preoperative therapy with immune checkpoint inhibitors), nivolumab has come to be recommended as the second-line treatment, based on demonstration, in a phase III trial, of its superiority over taxanes. Since the revision of the guidelines, nivolumab has been actively used in Japan for the treatment of unresectable or advanced esophageal cancer, and has shown some efficacy. There is a possibility that a significant improvement in prognosis will be reported in the future.
Conclusions
This report summarized the historical and current status of multimodal therapy for ESCC in Japan, based on clinical trials results carried out mainly by the JCOG.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-22/rc
Peer Review File: Available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-22/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-22/coif). Kazuo Koyanagi serves as an unpaid editorial board member of Annals of Esophagus from March 2020 to February 2022. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mariette C, Piessen G, Triboulet JP. Therapeutic strategies in oesophageal carcinoma: role of surgery and other modalities. Lancet Oncol 2007;8:545-53. [Crossref] [PubMed]
- Igaki H, Tachimori Y, Kato H. Improved survival for patients with upper and/or middle mediastinal lymph node metastasis of squamous cell carcinoma of the lower thoracic esophagus treated with 3-field dissection. Ann Surg 2004;239:483-90. [Crossref] [PubMed]
- Fukuda H. Development of cancer cooperative groups in Japan. Jpn J Clin Oncol 2010;40:881-90. [Crossref] [PubMed]
- Endo M, Yoshino K, Kawano T, et al. Clinicopathologic analysis of lymph node metastasis in surgically resected superficial cancer of the thoracic esophagus. Dis Esophagus 2000;13:125-9. [Crossref] [PubMed]
- Eguchi T, Nakanishi Y, Shimoda T, et al. Histopathological criteria for additional treatment after endoscopic mucosal resection for esophageal cancer: analysis of 464 surgically resected cases. Mod Pathol 2006;19:475-80. [Crossref] [PubMed]
- Makuuchi H, Shimada H, Mizutani K, et al. Clinical pathological analysis of surgically resected superficial esophageal carcinoma to determine criteria for deciding on treatment strategy. Diagn Ther Endosc 1997;3:211-20. [Crossref] [PubMed]
- Minashi K, Nihei K, Mizusawa J, et al. Efficacy of Endoscopic Resection and Selective Chemoradiotherapy for Stage I Esophageal Squamous Cell Carcinoma. Gastroenterology 2019;157:382-390.e3. [Crossref] [PubMed]
- Kato H, Sato A, Fukuda H, et al. A phase II trial of chemoradiotherapy for stage I esophageal squamous cell carcinoma: Japan Clinical Oncology Group Study (JCOG9708). Jpn J Clin Oncol 2009;39:638-43. [Crossref] [PubMed]
- Kato K, Igaki H, Ito Y, et al. Parallel-group controlled trial of esophagectomy versus chemoradiotherapy in patients with clinical stage I esophageal carcinoma (JCOG0502). In: Cannistra SA, Friedberg JW, Ballman KV, et al. editors. Meeting abstract: Proceeding of the Gastrointestinal Cancer Symposium; 2019 Feb 17-19; San Francisco, US. Alexandria; American Society of Clinical Oncology; 2019: P7.
- Cuschieri A, Shimi S, Banting S. Endoscopic oesophagectomy through a right thoracoscopic approach. J R Coll Surg Edinb 1992;37:7-11. [PubMed]
- Shichinohe T, Hirano S, Kondo S. Video-assisted esophagectomy for esophageal cancer. Surg Today 2008;38:206-13. [Crossref] [PubMed]
- Ozawa S, Ito E, Kazuno A, et al. Thoracoscopic esophagectomy while in a prone position for esophageal cancer: a preceding anterior approach method. Surg Endosc 2013;27:40-7. [Crossref] [PubMed]
- Akaishi T, Kaneda I, Higuchi N, et al. Thoracoscopic en bloc total esophagectomy with radical mediastinal lymphadenectomy. J Thorac Cardiovasc Surg 1996;112:1533-40; discussion 1540-1. [Crossref] [PubMed]
- Koyanagi K, Ozawa S, Tachimori Y. Minimally invasive esophagectomy performed with the patient in a prone position: a systematic review. Surg Today 2016;46:275-84. [Crossref] [PubMed]
- Kernstine KH, DeArmond DT, Karimi M, et al. The robotic, 2-stage, 3-field esophagolymphadenectomy. J Thorac Cardiovasc Surg 2004;127:1847-9. [Crossref] [PubMed]
- van Hillegersberg R, Boone J, Draaisma WA, et al. First experience with robot-assisted thoracoscopic esophagolymphadenectomy for esophageal cancer. Surg Endosc 2006;20:1435-9. [Crossref] [PubMed]
- Camarillo DB, Krummel TM, Salisbury JK Jr. Robotic technology in surgery: past, present, and future. Am J Surg 2004;188:2S-15S. [Crossref] [PubMed]
- Suda K, Ishida Y, Kawamura Y, et al. Robot-assisted thoracoscopic lymphadenectomy along the left recurrent laryngeal nerve for esophageal squamous cell carcinoma in the prone position: technical report and short-term outcomes. World J Surg 2012;36:1608-16. [Crossref] [PubMed]
- Torek F. The first successful case of resection of the thoracic portion of the oesophagus for carcinoma. Surg Gynecol Obstet 1913;16:614-7.
- Fujita H. History of lymphadenectomy for esophageal cancer and the future prospects for esophageal cancer surgery. Surg Today 2015;45:140-9. [Crossref] [PubMed]
- Sannohe Y, Hiratsuka R, Doki K. Lymph node metastases in cancer of the thoracic esophagus. Am J Surg 1981;141:216-8. [Crossref] [PubMed]
- Kato H, Tachimori Y, Mizobuchi S, et al. Cervical, mediastinal, and abdominal lymph node dissection (three-field dissection) for superficial carcinoma of the thoracic esophagus. Cancer 1993;72:2879-82. [Crossref] [PubMed]
- Baba M, Aikou T, Yoshinaka H, et al. Long-term results of subtotal esophagectomy with three-field lymphadenectomy for carcinoma of the thoracic esophagus. Ann Surg 1994;219:310-6. [Crossref] [PubMed]
- Fujita H, Kakegawa T, Yamana H, et al. Mortality and morbidity rates, postoperative course, quality of life, and prognosis after extended radical lymphadenectomy for esophageal cancer. Comparison of three-field lymphadenectomy with two-field lymphadenectomy. Ann Surg 1995;222:654-62. [Crossref] [PubMed]
- Ando N, Ozawa S, Kitagawa Y, et al. Improvement in the results of surgical treatment of advanced squamous esophageal carcinoma during 15 consecutive years. Ann Surg 2000;232:225-32. [Crossref] [PubMed]
- Altorki N, Kent M, Ferrara C, et al. Three-field lymph node dissection for squamous cell and adenocarcinoma of the esophagus. Ann Surg 2002;236:177-83. [Crossref] [PubMed]
- Lerut T, Nafteux P, Moons J, et al. Three-field lymphadenectomy for carcinoma of the esophagus and gastroesophageal junction in 174 R0 resections: impact on staging, disease-free survival, and outcome: a plea for adaptation of TNM classification in upper-half esophageal carcinoma. Ann Surg 2004;240:962-72; discussion 972-4. [Crossref] [PubMed]
- Fang WT, Chen WH, Chen Y, et al. Selective three-field lymphadenectomy for thoracic esophageal squamous carcinoma. Dis Esophagus 2007;20:206-11. [Crossref] [PubMed]
- Kato H, Watanabe H, Tachimori Y, et al. Evaluation of neck lymph node dissection for thoracic esophageal carcinoma. Ann Thorac Surg 1991;51:931-5. [Crossref] [PubMed]
- Shang QX, Chen LQ, Hu WP, et al. Three-field lymph node dissection in treating the esophageal cancer. J Thorac Dis 2016;8:E1136-49. [Crossref] [PubMed]
- Ma GW, Situ DR, Ma QL, et al. Three-field vs two-field lymph node dissection for esophageal cancer: a meta-analysis. World J Gastroenterol 2014;20:18022-30. [Crossref] [PubMed]
- Akakura I, Nakamura Y, Kakegawa T, et al. Surgery of carcinoma of the esophagus with preoperative radiation. Chest 1970;57:47-57. [Crossref] [PubMed]
- Kasai M. Surgical treatment for carcinoma of the esophagus. J Jpn Surg Soc 1980;81:845-53.
- Iizuka T, Ide H, Kakegawa T, et al. Preoperative radioactive therapy for esophageal carcinoma. Randomized evaluation trial in eight institutions. Chest 1988;93:1054-8. [Crossref] [PubMed]
- A comparison of chemotherapy and radiotherapy as adjuvant treatment to surgery for esophageal carcinoma. Japanese Esophageal Oncology Group. Chest 1993;104:203-7. [Crossref] [PubMed]
- Ando N, Iizuka T, Kakegawa T, et al. A randomized trial of surgery with and without chemotherapy for localized squamous carcinoma of the thoracic esophagus: the Japan Clinical Oncology Group Study. J Thorac Cardiovasc Surg 1997;114:205-9. [Crossref] [PubMed]
- Ando N, Iizuka T, Ide H, et al. Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: a Japan Clinical Oncology Group Study--JCOG9204. J Clin Oncol 2003;21:4592-6. [Crossref] [PubMed]
- Kleinberg L, Forastiere AA. Chemoradiation in the management of esophageal cancer. J Clin Oncol 2007;25:4110-7. [Crossref] [PubMed]
- Ando N, Kato H, Igaki H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol 2012;19:68-74. [Crossref] [PubMed]
- Hirao M, Ando N, Tsujinaka T, et al. Influence of preoperative chemotherapy for advanced thoracic oesophageal squamous cell carcinoma on perioperative complications. Br J Surg 2011;98:1735-41. [Crossref] [PubMed]
- Hara H, Tahara M, Daiko H, et al. Phase II feasibility study of preoperative chemotherapy with docetaxel, cisplatin, and fluorouracil for esophageal squamous cell carcinoma. Cancer Sci 2013;104:1455-60. [Crossref] [PubMed]
- Nakamura K, Kato K, Igaki H, et al. Three-arm phase III trial comparing cisplatin plus 5-FU (CF) versus docetaxel, cisplatin plus 5-FU (DCF) versus radiotherapy with CF (CF-RT) as preoperative therapy for locally advanced esophageal cancer (JCOG1109, NExT study). Jpn J Clin Oncol 2013;43:752-5. [Crossref] [PubMed]
- Shah MA, Kojima T, Hochhauser D, et al. Efficacy and Safety of Pembrolizumab for Heavily Pretreated Patients With Advanced, Metastatic Adenocarcinoma or Squamous Cell Carcinoma of the Esophagus: The Phase 2 KEYNOTE-180 Study. JAMA Oncol 2019;5:546-50. [Crossref] [PubMed]
- Jiang Y, Lo AWI, Wong A, et al. Prognostic significance of tumor-infiltrating immune cells and PD-L1 expression in esophageal squamous cell carcinoma. Oncotarget 2017;8:30175-89. [Crossref] [PubMed]
- Guo W, Wang P, Li N, et al. Prognostic value of PD-L1 in esophageal squamous cell carcinoma: a meta-analysis. Oncotarget 2018;9:13920-33. [Crossref] [PubMed]
- Qu HX, Zhao LP, Zhan SH, et al. Clinicopathological and prognostic significance of programmed cell death ligand 1 (PD-L1) expression in patients with esophageal squamous cell carcinoma: a meta-analysis. J Thorac Dis 2016;8:3197-204. [Crossref] [PubMed]
- Kato K, Cho BC, Takahashi M, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2019;20:1506-17. [Crossref] [PubMed]
- Yamamoto S, Kato K, Daiko H, et al. Feasibility study of nivolumab as neoadjuvant chemotherapy for locally esophageal carcinoma: FRONTiER (JCOG1804E). Future Oncol 2020;16:1351-7. [Crossref] [PubMed]
- Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA 1999;281:1623-7. [Crossref] [PubMed]
- Kato K, Muro K, Minashi K, et al. Phase II study of chemoradiotherapy with 5-fluorouracil and cisplatin for Stage II-III esophageal squamous cell carcinoma: JCOG trial (JCOG 9906). Int J Radiat Oncol Biol Phys 2011;81:684-90. [Crossref] [PubMed]
- Ito Y, Takeuchi H, Ogawa G, et al. A single-arm confirmatory study of definitive chemoradiotherapy (dCRT) including salvage treatment in patients (pts) with clinical (c) stage II/III esophageal carcinoma (EC) (JCOG0909). J Clin Oncol 2018;36:4051. [Crossref]
- Shinoda M, Ando N, Kato K, et al. Randomized study of low-dose versus standard-dose chemoradiotherapy for unresectable esophageal squamous cell carcinoma (JCOG0303). Cancer Sci 2015;106:407-12. [Crossref] [PubMed]
- Yokota T, Kato K, Hamamoto Y, et al. Phase II study of chemoselection with docetaxel plus cisplatin and 5-fluorouracil induction chemotherapy and subsequent conversion surgery for locally advanced unresectable oesophageal cancer. Br J Cancer 2016;115:1328-34. [Crossref] [PubMed]
- Terada M, Hara H, Daiko H, et al. Phase III study of tri-modality combination therapy with induction docetaxel plus cisplatin and 5-fluorouracil versus definitive chemoradiotherapy for locally advanced unresectable squamous-cell carcinoma of the thoracic esophagus (JCOG1510: TRIANgLE). Jpn J Clin Oncol 2019;49:1055-60. [Crossref] [PubMed]
- Kataoka K, Tsushima T, Mizusawa J, et al. A randomized controlled Phase III trial comparing 2-weekly docetaxel combined with cisplatin plus fluorouracil (2-weekly DCF) with cisplatin plus fluorouracil (CF) in patients with metastatic or recurrent esophageal cancer: rationale, design and methods of Japan Clinical Oncology Group study JCOG1314 (MIRACLE study). Jpn J Clin Oncol 2015;45:494-8. [Crossref] [PubMed]
Cite this article as: Kanamori K, Koyanagi K, Ozawa S, Yamamoto M, Ninomiya Y, Yatabe K, Higuchi T, Tajima K. Multimodal therapy for esophageal squamous cell carcinoma according to TNM staging in Japan—a narrative review of clinical trials conducted by Japan Clinical Oncology Group. Ann Esophagus 2023;6:32.


