
Modern evaluation of esophageal function in the gastrointestinal motility laboratory: a narrative review
Introduction
The gastrointestinal (GI) motility laboratory has been transformed over the last decade, modernizing its approaches to improve the evaluation of esophageal motility disorders and gastroesophageal reflux disease (GERD). Novel applications and protocols for established testing modalities as well as new technologies allow for a more nuanced and actionable evaluation of patients with symptoms of esophageal dysfunction. Dysphagia, heartburn, chest pain, and certain extra-esophageal symptoms can all be better assessed and treated with these advances. This article aims to review the current state of functional esophageal evaluation in the GI motility laboratory, highlighting updates in established modalities along with new technologies (Table 1). For this narrative review, we also reviewed literature searches made using PubMed for years 2010 to April 2021. Evaluations of esophageal motility, including esophageal manometry and endoscopic functional luminal imaging probe (EndoFLIP), will be covered first followed by the tools for the evaluation of GERD. We present the following article in accordance with the Narrative Review reporting checklist (available at: https://aoe.amegroups.com/article/view/10.21037/aoe-21-36/rc).
Table 1
| Esophageal manometry |
| Water perfused catheter |
| Solid state catheter |
| High-resolution manometry |
| Esophageal pH monitoring |
| pH monitoring catheter |
| Impedance-pH monitoring catheter |
| Wireless pH capsule |
| Endoscopic functional luminal imaging probe (EndoFLIP) |
Esophageal motility evaluation
The goals of esophageal motility testing are to assess esophageal motor function and to identify any patterns of abnormal muscular activity. Identifying and characterizing esophageal dysmotility allows for appropriate treatment selection and can provide important prognostic information for patients and referring physicians. Advances in technology have improved our ability, not only to measure esophageal motor abnormalities, but also to better understand the relationship between these abnormalities and patient symptoms. Esophageal manometry remains the gold standard for evaluation of esophageal motility, now using high-resolution recordings with closely spaced recording channels to assess global esophageal function. Esophageal EndoFLIP is a newer technology that is becoming an important adjunct tool especially for sphincter evaluation to assess compliance and cross-sectional areas (CSAs).
High resolution esophageal manometry with impedance (HREMI)
Esophageal manometry is useful in the patients with nonobstructive dysphagia and in the evaluation of patients with refractory reflux symptoms being considered for anti-reflux surgery (ARS). Esophageal manometry may specifically diagnose achalasia, diffuse esophageal spasm, and motility disorders associated with systemic disorders, particularly scleroderma. In addition, other disorders such as esophagogastric junction outlet obstruction (EGJOO) associated with dysphagia, and Jackhammer Esophagus associated with chest pain may be detected.
HREMI is now the standard evaluation of esophageal motor function (1). HREMI utilizes catheters with 36 sensors spaced 1 cm apart which provide pressure measurements along the entire length of the esophagus from the oropharynx to the proximal stomach. These measurements are translated into colorful spatiotemporal topography plots via esophageal pressure topography (EPT) (Figure 1) (2). Interpretation of these pressure readings and EPT plots, using the Chicago Classification (now in its 4th iteration) as a standardized algorithm, provides insight into the function of the upper esophageal sphincter (UES), the esophageal body, the LES, and the esophagogastric junction (EGJ) complex (1). The value of HREMI in the evaluation of esophageal dysmotility is well established. This article reviews the current standard approach to HREMI and also aims to review novel metrics and methods that allow utilization of HREMI beyond investigation of dysphagia. Most promising amongst these applications are metrics for predicting response to therapies for GERD and detection of esophageal mucosal abnormalities—particularly esophagitis and Barrett’s esophagus (BE).
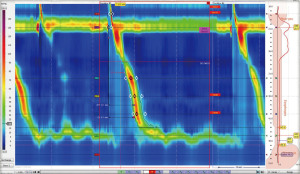
HREMI procedure
The HREMI study entails the protocol suggested by the current Chicago Classification 4.0 (Table 2). This starts with, after calibration of the catheter, insertion of the recording catheter nasally, passing the catheter into the stomach so that the recording ports span from the oropharynx to the proximal stomach. After a 3-minute equilibration of the catheter to body temperature, the patient takes several deep breaths to ensure one sees the decrease in intrathoracic pressure and increase in the intragastric pressure ensuring passage of the catheter crossing the diaphragm into the stomach, which occasionally is difficult in patients with achalasia or large hiatal hernias. The protocol entails an initial 30 second supine landmark period without patient swallowing to record basal pressures. Then, the patient swallows 5 cc of saline every 30 seconds for 10 swallows to assess esophageal peristalsis with appropriate UES and LES relaxation. Saline is used to help measure the impedance along the esophagus to help assess actual fluid flow. This is followed by the multiple rapid swallows (MRS) protocol in which the patient swallows 2 cc of saline every 5 seconds. We then have the patient perform a 6-inch leg lift (straight leg raise test) which increases both LES and crural diaphragm (CD) pressure to help assess for hiatal hernia. Then, the patient sits up, and after equilibration ensuring the catheter is properly positioned, a 30-second upright landmark period without swallows is recorded. This is followed by the patient swallowing 5 cc of saline every 30 seconds for 5 swallows to assess esophageal contractility in the upright position. Then, a final provocation test is performed. This could be MRS upright to assess esophageal and LES function or the rapid drink challenge test to assess the LES for relaxation when this is not clear on the baseline study. If there is the concern for rumination, a prolonged recording in the upright position for 30–60 minutes can be performed with the patient consuming their incriminating foods or eating graham crackers followed by apple sauce, looking for gastric contractions followed by regurgitation—signs of rumination. At the end of the study, the catheter is withdrawn while still recording to measure atmospheric pressure.
Table 2
| Patient position | Maneuver |
|---|---|
| Sitting upright | Calibrate catheter |
| Insert catheter nasally into stomach | |
| Supine | Equilibrate catheter to body temperature for 3 minutes |
| Deep breath | |
| Landmark for 30 seconds | |
| 10 saline swallows, 30 seconds apart | |
| Multiple rapid swallow | |
| Leg lift | |
| Sitting upright | Equilibrate catheter |
| Landmark for 30 seconds | |
| 5 saline swallows, 30 seconds apart | |
| Provocative tests upright | Multiple rapid swallow or rapid drink challenge |
| Optional: rumination protocol | |
| Finishing the study | Remove catheter |
| Record atmospheric pressure |
HREMI, high resolution esophageal manometry with impedance.
HREMI test analysis
Pressure topography analysis
Analysis of the procedure involves first assessing the GEJ, then the esophageal body, then the UES (Table 3). The basal GEJ pressure is measured during the supine landmark study; assessing for two high pressure zones suggesting a hiatal hernia—distal CD phasic contractions and proximal tonic LES pressure. Relaxation of the LES is determined for each of the 10 swallows, calculating the Integrated Residual Pressure (IRP)—the median value of the lowest 4 seconds of the GEJ pressure after the swallow. Normal is <15 mmHg for the Medtronic system. If the IRP is >15 mmHg, this suggests impaired LES relaxation pointing towards achalasia if there is no normal peristalsis of the esophageal body. If the IRP is >15 mmHg with peristalsis, this brings up Esophagogastric Outlet Obstruction (EGJOO). The updated Chicago 4.0 criteria for EGJOO also requires elevated IRP >12 mmHg in the upright position along with another confirmatory test by radiology or EndoFLIP. Achalasia is divided into types I, II, III depending on the esophageal body (Figure 2). In achalasia type I, there are no esophageal contractions in response to swallows. In achalasia type II, there are simultaneous contractions that are isobaric throughout the esophagus, resembling Roman pillars. In achalasia type III, there are often forceful spastic simultaneous contractions that are not isobaric. For achalasia type III, the HREMI can be used to guide the length of the esophagomyotomy as the myotomy should entail incision of the esophageal smooth muscle up the esophageal body for the length of the high-pressure esophageal finding on esophageal manometry.
Table 3
| Parameter | Description |
|---|---|
| Pressure topography | |
| Esophagogastric junction (EGJ) | Basal EGJ pressure during landmark |
| Presence of hiatal hernia | |
| Integrated residual pressure (IRP) on swallowing | |
| EGJ-contractile integral (CI) | |
| Esophageal body | Response to swallows: peristalsis, simultaneous, fragmented, non-transmitted |
| Distal contractile integral (DCI): amplitude of esophageal contraction | |
| Distal latency (DL), marker for peristalsis | |
| Upper esophageal sphincter | Basal pressure |
| Response to swallowing (residual pressure) | |
| Impedance | |
| Esophageal body | Esophageal bolus clearance |

The esophageal body contractions in response to swallows is assessed for peristalsis and integrity of the contractile wave. The distal contractile integral (DCI) assesses the amplitude of the contraction wave. The distal latency (DL) is used as an index of simultaneous contractions.
The UES is assessed for basal UES pressure during the landmark phase, and degree of relaxation with swallows.
Impedance analysis
Bolus clearance of the swallowed saline water is the main analysis assessed using impedance. The impedance values monitor the flow of the saline bolus down the esophagus and into the stomach. This can be performed displaying the actual impedance values. Alternatively, the flow is usually assessed with topography mode where the saline bolus is colored purple whereas the esophageal contractile wave is colored yellow to red. Reflux can also be detected during the HREMI study with the impedance showing that after the bolus enters the stomach, it refluxes back in the esophagus.
New metrics in HREMI
EGJ morphology and EGJ contractile integral
Anatomic alteration and physiologic dysfunction of the EGJ complex is a primary pathogenic factor in GERD (3), an evaluation of its structural and functional integrity using novel HREMI metrics can complement multichannel intraluminal impedance-pH (MII-pH) data and assist in this determination. Both EGJ morphology (anatomic relationship between the CD and the LES] and the EGJ-contractile integral (EGJ-CI, a measure of EGJ contractility in relation to respiration) have been shown to correlate with response to proton pump inhibitor (PPI) therapy as well as ARS (Figure 3) (4-6).
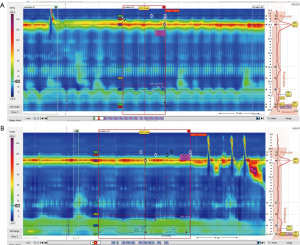
EGJ morphology is determined manometrically and is defined as one of three sub-types: type I—normal, with the CD superimposed over the LES with respiratory inversion point (RIP) proximal to the complex; type II—LES-CD separation with the RIP located proximal to the CD, and type III/C—LES-CD separation with the RIP located proximal to the LES (5). The normal type I EGJ morphology has been noted to predict non-response to treatment for GERD while the abnormal types II and III (reflecting the presence of a hiatal hernia) predict response to treatment for GERD (2).
EGJ-CI is a metric calculated by using the DCI function across the EGJ for 3 respiratory cycles and dividing it by the duration of those 3 cycles (7). Normal EGJ-CI has been shown to be independently associated with non-response while low EGJ-CI values, suggesting EGJ-complex dysfunction, were associated with response (2,6,7). The threshold for normal vs. low EGJ-CI remains to be determined. Prior evaluations suggested 39 mmHg·cm, however more recent guidelines have recommended 25 mmHg·cm as a more useful cutoff (5,7).
Baseline impedance (BI)
Esophageal BI is another novel HREMI metric that can help predict response to anti-reflux therapies in GERD. Impedance is a measure of resistance to current flow between electrical poles—two catheter-based electrodes in the case esophageal impedance technology. Compared to normal mucosa, GERD induced esophagitis features altered mucosal integrity via dilation of intracellular spaces, allowing increased flow of electrolyte fluid around the cells and better conduction of electrical current, resulting in lower impedance (8-11). Via this interplay between mucosal integrity and impedance, esophageal BI can indicate the presence of true pathologic acid exposure (8,12) as average BI has been shown to be significantly lower in true GERD patients compared to those with non-acid exposure syndromes (i.e., functional heartburn) (13). A metric extracted from 24-hour MII-pH testing that relies on this principle, the mean nocturnal baseline impedance (MNBI), has been shown to predict patient response to anti-reflux therapies with lower levels (<2,292 Ω) predictive of response (13,14). Recently, BI as determined by HREMI (BI-HREMI) has been shown to correlate well with MNBI and thus can provide similar predictive support without the burden of a 24-hour MII-pH (9). In addition to acting as a surrogate for MNBI, BI-HREMI can predict the presence and extent of BE as well. BE has been shown to have lower distal esophageal BI-HREMI than esophagitis, suggesting a BI-HREMI continuum with BE lower than esophagitis and esophagitis lower than normal mucosa (8).
Provocative tests
(I) MRS
In many patients, however, the HREM study appears normal. In these patients, provocative maneuvers have been attempted to bring out esophageal motility disturbances. One maneuver is the MRS technique, which has been shown to contribute to the assessment of motor function (15). The MRS consists of swallowing 2 mL of water every 2 seconds for 5 consecutive swallows. The MRS is used to assess inhibitory swallowing mechanisms and esophageal peristaltic reserve. The MRS elicits central and peripheral neuronal inhibitions in the LES, esophageal body, and EGJ during the period of deglutitive inhibition; it is normally followed by a period of excitatory contraction of the esophageal body and subsequent reestablishment of the LES tone. An abnormal response will have incomplete inhibition of peristalsis characterized by esophageal body contraction during the inhibitory phase, failure of LES relaxation, and/or diminished or absent peristalsis after the MRS. The MRS has been suggested to evaluate candidacy for fundoplication in patients with GERD. Patients with weak esophageal body response compared to their baseline single swallow were found to be predictive of late postoperative dysphagia (16).
(II) Rapid drinking challenge (RDC)
Another provocative swallowing technique is the RDC, with the patient drinking 200 mL of water quickly but at a rate determined by the patient. RDC has been reported to increase sensitivity for detecting EGJ dysfunction (17). The GEJ should relax to an IRP of <12.
(III) Rumination protocol
If there is the concern that the patient has rumination, this can be evaluated during the esophageal manometry. Before the procedure, the patient is asked what foods or liquids bring on the rumination, and asked to bring this in. After the baseline esophageal manometry, a prolonged recording in the upright position for 30–60 minutes can be performed with the patient consuming their incriminating foods or eating graham crackers followed by apple sauce. The study is analyzed by looking for gastric contractions followed by regurgitation—signs of rumination.
Esophageal EndoFLIP
The functional luminal imaging probe (FLIP) has emerged as a valuable tool in the evaluation of esophageal function and pathology. Utilizing a catheter with a distal overlying balloon fitted with impedance planimetry electrodes (Medtronic Inc., Shoreview, MN, USA), EndoFLIP measures esophageal luminal diameter and corresponding distension pressures during volumetric distention (18,19). This data is displayed as a 3D image of the esophageal lumen with a corresponding FLIP panometry plot [similar to pressure topography plots produced in high-resolution manometry (HREMI) studies].
EndoFLIP is used to provide information on the LES function including diameter, cross sectional area, distensibility and compliance (Figure 4). For this, the smaller 8-cm long balloon is used. Esophageal FLIP can provide information on wall stiffness [helpful for patients with possible eosinophilic esophagitis (EoE)], and esophageal motility that can aid in diagnosis and help guide management decisions (19-21). For this, the longer 16-cm balloon is usually used.
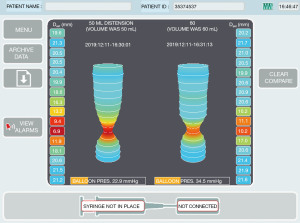
Achalasia and EGJOO in particular are sensitively identified using EndoFLIP (18,22,23). esophagogastric junction-distensibility index (EGJ-DI) <2.8 mm2/mmHg reliably indicates EGJ dysfunction and esophageal contractility pattern can then be used to differentiate achalasia (and its sub-types) from EGJOO. In fact, the Chicago Classification v4.0 recommends EndoFLIP [and/or timed barium esophagram (TBE)] be used to confirm presence of suspected EGJOO detected on HREMI prior to any LES directed therapy (5). Pre-operative and intra-operative EndoFLIP may also have utility in predicting response to LES directed therapy, namely pneumatic dilation, surgical myotomy, and per-oral endoscopic myotomy (POEM) (1,4,24,25).
Key metrics measured by EndoFLIP include EGJ-DI, maximum EGJ diameter, and intraluminal distensibility plateau (DP) as well as luminal contractility patterns. EGJ-DI, a measure of EGJ distensibility, is measured as the CSA at the EGJ divided by median intra-balloon pressure. DP, a measure of luminal distensibility and wall stiffness, is calculated via a polynomial regression technique using esophageal body diameter-pressure relationships. Carlson et al. determined that normal values for these metrics (at 60 mL balloon distention) include EGJ-DI >2.8 mm2/mmHg, maximum EGJ diameter ≥18 mm, and DP ≥18 mm (1,19). Esophageal contractions elicited by balloon distention are determined to be antegrade (caudad) or retrograde (cephalad) with patterns including repetitive antegrade contractions (RAC), repetitive retrograde contractions (RRC), diminished-disordered contractile response (DDCR) and absent contractility. The RAC is the contractile pattern present in normal studies (21).
Utilizing these metrics, EndoFLIP has been shown to provide reliable real time evaluation of esophageal motility at the time of endoscopy (22). Comparable to the algorithmic analysis of esophageal motility outlined in the Chicago Classification focusing on EGJ functionality and then esophageal peristalsis (5), EndoFLIP findings can be organized based on EGJ distensibility (EGJ-DI) and distention induced contractility patterns. FLIP can reliably predict benign HREMI studies as well as detect abnormal esophageal motility at the time of endoscopy, complementing results of prior HREMI or dictating which patients would benefit from manometric assessment (20,22).
Esophageal wall stiffness, abnormal in EoE and other remodeling conditions of the esophagus, can also be evaluated using EndoFLIP. In EoE patients, decreased esophageal distensibility as indicated by lower DP has been shown to be associated with increased ring/stricture severity, need for dilation, and risk of food impaction whereas severity of mucosal eosinophilia was not predictive of these important outcomes (26). EndoFLIP may thus prove to be a more useful tool for monitoring disease activity in EoE than standard upper endoscopy with biopsy, especially considering inconsistency in mucosal sampling (19).
EndoFLIP has become an important tool in the armamentarium for evaluation of esophageal functionality. Assessment of the LES and EGJ is currently its most robust application, however, data supporting its utility in evaluation of esophageal body peristalsis and stiffness is growing. Further refinement of its place in pre- and post-treatment evaluations for various esophageal disorders as well as diagnostic utility for conditions primarily involving the esophageal body are on the horizon.
Gastroesophaegal reflux evaluation
GERD is one the most common gastrointestinal conditions worldwide. Factors contributing to development of GERD include EGJ dysfunction, ineffective acid and bolus clearance, increase gastric pressure, and anatomical changes leading to EGJ weakening (e.g., hiatal hernia) (27). Reflux during transient lower esophageal sphincter relaxation (tLESR) has been recognized as an important mechanism, in addition to presence of low LES pressure and presence of a hiatal hernia (28).
The most commonly used approach to patients with typical GERD symptoms (heartburn and regurgitation) is empirical treatment with PPIs. However, the response to PPI is neither sensitive nor specific. In an analysis of data from the multinational DIOMOND study, positive response to the PPI test was observed in 69% of patients with actual GERD and in 51% of those without GERD (29). Furthermore, GERD can present with a wide range of less typical symptoms which include dysphagia, chest pain, water brash, burping, hiccups, nausea, and vomiting (30). Several questionnaires have been developed to diagnose GERD but their use by even experienced gastroenterologists showed 70% sensitivity and 67% specificity compared to endoscopy and pH studies (31).
The role of endoscopy is limited in diagnosing GERD as up to 85% of patients with typical GERD symptoms have non-erosive esophagitis reflux disease (NERD), and should mainly be used to evaluate patients with prolonged GERD symptoms despite optimal PPI therapy, alarm features, and in those with symptoms of GERD complications including BE, peptic strictures, or malignancy (32,33).
Ambulatory esophageal pH monitoring studies remain the standard for the diagnosis of GERD (34), particularly in those patients with normal endoscopy, atypical symptoms, and before ARS (14). Prolonged pH measurement has a high sensitivity (77–100%) and high specificity (85–100%) of detecting excessive esophageal acid exposure in patients with endoscopically proven esophagitis compared to normal patients (34,35). Current pH monitoring modalities include pH catheters, MII-pH, and wireless pH monitor (Table 1) (35).
Multichannel impedance-pH monitor
The MMII-pH study records both pH and impedance data. This allows for assessment of the reflux severity (reflux burden), determination of the relationship between symptom occurrence and reflux episodes, and distinguishing between acidic and non-acidic reflux episodes (32,36).
The role of MII-pH is to detect the presence of gastroesophageal reflux in patients with persistent typical GERD symptoms despite adequate acid-suppression without endoscopic evidence of GERD, patients with atypical GERD symptoms, and patients being considered for ARS (37). Testing is typically performed on PPI therapy for typical GERD symptoms that are refractory to medical therapy while it is typically performed off PPI therapy in those with atypical symptoms (36).
Catheter characteristics and placement
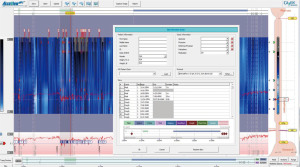
The MII-pH probe is a 2-mm catheter that contains ring electrodes positioned 3, 5, 7, 9, 15 and 17 cm from GEJ. With this setup, intraluminal pH is measured 5 cm above the LES (esophageal pH). We employ a catheter that also measures gastric pH, 10 mm below the LES (38). Figure 5 shows a typical MII-pH setup.
The catheter, after proper calibration, is inserted trans-nasally until the most distal pH sensor is in the stomach and the more proximal pH sensor is positioned at 5 cm above the LES as determined by esophageal manometry (35). High-resolution esophageal manometry is often used to determine the position of the LES as other methods are not as accurate (38,39). After confirming appropriate positioning, the recording of pH and impedance data begins and usually continues for 24 hours. Patients are provided with a paper diary and are asked to record their symptoms, meal episodes, position (supine vs. upright), and acid-suppressor intake while also pressing the corresponding buttons on the recorder device. Moreover, they are encouraged to continue with their routine eating and drinking behaviors and usual daily activities throughout the study (35). The patients return the next day to have the catheter removed and diary information is verified (Figure 6).
Interpretation
pH analysis
Total acid exposure time (AET), which is the percentage of total recording time that the distal esophageal pH is less than 4, is the most reproducible and validated metric to define abnormal reflux burden. It is also the most reliable predictor of therapeutic outcome (32,36,40,41). Valid results require at least 16 hours of recording and mealtimes need to be excluded to avoid measuring acidic events from the ingested meal (36). AET is calculated for the duration of the study as well as calculated separately for supine and upright periods. Abnormal AET during supine position could indicate a disrupted EGJ barrier function with low LES pressure as tLESRs is generally suppressed during sleep (42). Abnormal AET >4.5% is often used for evaluation. However, the Lyon Consensus, an international consensus document on GERD, recommends AET >6% off PPIs as the cutoff for positive diagnosis of GERD and AET <4% to be physiologic (normal) in the absence of endoscopic evidence of GERD (14). AET results of 4–6% are considered borderline and their interpretation requires additional clinical correlation. Often these values are used whether the study is performed off or on PPI therapy. More refined guidelines for interpreting studies performed on PPI are needed, both for impedance episodes and AETs (43,44). For normal subjects taking PPI daily, the upper limit of AET is 2.5% and normal subjects taking PPI BID, the upper limit of esophageal acid exposure decreases to 1.3%.
One pitfall of pH interpretation is inaccurate recording of meal episodes by patients as these periods should be excluded from the study, otherwise acidic meal consumptions can falsely increase the total AET. The meal times need to be identified during analysis and excluded (45). Catheter misplacement or dislodging can also lead to inaccurate results. These could be minimized by inspecting the condition and position of the catheter when the patient returns the next day. One drawback of MII-pH study is that some patients may struggle to eat and behave normally with an esophageal catheter in place which leads to underestimation of reflux severity. Furthermore, day-to-day variation is often observed in many patients (46). For this reason, if the suspicion for GERD remains high despite a negative 24-hour pH study, an extended 4-day wireless pH capsule study could be considered which has shown to improve diagnostic yield (36,47).
Impedance analysis
Along the MII-pH probe, an electric current is generated between each pair of electrodes and the impedance to current flow is measured (37). An ion-dense liquid bolus when passing along the electrodes leads to drop in the impedance. On the other hand, gas (e.g., during belching) which is less ion-dense leads to an increase in the impedance (48).
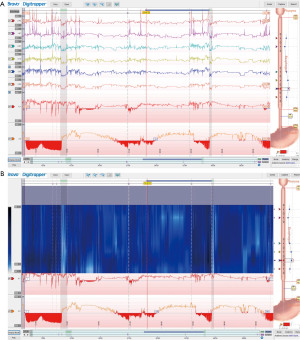
Impedance analysis can detect the direction of flow regardless of the acidity and thus differentiate between swallows and reflux episodes. Further, it can characterize these events as either liquid, gas, or mixed (35). Additionally, adding impedance analysis to pH monitoring helps to identify and distinguish acidic (pH <4), weakly acidic (pH 4–7), and alkaline (pH >7) reflux episodes. Proximal extent of reflux can be assessed as the electrodes are distributed along the catheter; this can be helpful in patients with symptoms of hoarseness, sore throat and coughing which might be related to reflux. Additional information provided by impedance monitoring such as bolus exposure time, bolus clearance time provide further clinical context, however interpretation of these data points remains challenging as there is no consensus among experts regarding expected normal values (49). Figure 7 shows a sample MII-pH tracing.
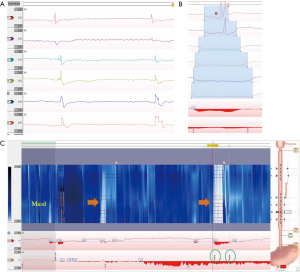
The Wingate consensus recently defined a reflux episode detected by impedance as a 50% decrease in impedance lasting for at least 4 seconds each in distal 2 impedance channels with retrograde propagation (see Figure 8) (45). The Lyon consensus proposed that >80 reflux episodes in 24 hours should be considered abnormal, <40 episodes are probably normal physiologically, and reflux between 40–80 episodes is borderline (14). This parameter at this time is still considered as adjunctive data when AET is inconclusive (between 4–6%). It is important to note that all reflux episodes need to be analyzed manually as the automated analysis often is inaccurate and overestimates the reflux episodes (45,50).
Symptom association analysis
An integral component of pH monitoring studies is determining temporal relationship between symptom events and reflux episodes (51). Patients are asked about the most bothersome symptom which is then assessed during the study. An optional cough detector can also compliment the study in patients presenting with cough, and recently used in clinical research studies (36). Various methods were developed to assess the symptom-reflux relationship, but the most commonly used ones are symptom index (SI) and symptom association probability (SAP) (34). The SI is a ratio of the percentage of symptoms within a 2-minute window of a reflux episode, which if >50% is considered positive. It does not account for the total number of reflux episodes and it may be abnormal by chance, especially with few symptom episodes. The SAP takes into account 2-minute periods with and without reflux episodes and with and without symptom events and applies a Fisher’s exact test to see if there is a statistical difference for symptom episodes being present during reflux compared to without reflux. A P value <0.05 (SAP >95%) is considered positive symptom association (52). At least 3 symptoms during the study are required for a reliable symptom association analysis, and SI (measuring the effect size), and SAP (measuring the probability) are not comparable but rather complimentary (32,33). These calculations rely on timely and accurate symptom reporting by patients, so it is important to carefully explain the instructions and review the diary to ensure reliable results (36,53).
Novel metrics in MII-pH studies
In recent years, new parameters have been developed, which include mucosal impedance and the post-reflux swallow-induced peristaltic wave (PSPW) index. These parameters correlate well with GERD diagnosis and predicting outcome and response to therapy, particularly in patients with borderline AET (4–6%) (13,32,54-56).
MNBI
BI has been shown to be a sign of mucosal integrity via changes in intracellular spaces and tight junctions (27,57). Lower BI values indicate compromise in the integrity of these tight junctions which is thought to happen in patients with GERD (58,59). BI can be measured by various methods including high-resolution esophageal manometry, an endoscopically placed mucosal probe, or esophageal balloon with electrode strips (32). Impedance data obtained from MII-pH has been used to obtain the MNBI. Impedance values can be through swallowed fluid or reflux contents. When there is no liquid in the esophagus, as should be during sleeping, the current between electrodes (impedance) travels through the mucosa and provides a measure of mucosal integrity. These measurements are taken from the nocturnal periods as the interference from swallows and reflux events is minimal (60). The mean impedance levels are obtained from the most distal channel (3 cm above LES) during three discrete 10-miute periods (separated by one hour) during nighttime supine positioning. MNBI compared to analysis of more than 6 hours impedance showed a high interclass correlation (ICC =0.99) and BI levels were lower in GERD patients who responded to PPI compared to both non-responders and healthy volunteers (58). Various studies have shown lower MNBI levels in patients with erosive and non-erosive esophagitis, as well as reflux hypersensitivity compared with normal controls and even functional heartburn (54,55,60,61). A prospective study by Frazzoni et al. demonstrated that MNBI has a 91% sensitivity and 86% specificity for detecting non-erosive esophagitis and 72% sensitivity and 86% specificity to detect erosive esophagitis with best cutoff value of 2,292 Ω (AUC =0.876) (54). In another study, they showed improvement of MNBI after ARS (P=0.022) (55). In a separate case-control study, patients with functional heartburn who had >50% response to PPIs, and patients with hypersensitive esophagus had lower MNBI compared to non-responders and healthy volunteers (P<0.001) (62).
PSPW index
Another novel metric measured with MII-pH is the PSPW index. Under normal physiologic conditions, reflux episodes stimulate stretch receptors in the esophageal wall which in turn trigger secondary peristaltic waves intended to clear the refluxate (32). Additional vagally mediated reflexes also triggered by reflux episodes produce primary voluntary swallows intended to neutralize an acidic esophagus via alkaline saliva. This entire process is called “chemical clearance” and these swallows, which occur within 30 seconds of a reflux episode, are PSPWs (32,63). The PSPW index is a metric derived by dividing the total number of PSPWs during the test period by the total number of reflux events. The aforementioned studies by Frazzoni et al. reported that a PSPW index under 61% (AUC =0.977) detected erosive and non-erosive esophagitis with 100% and 89% sensitivity respectively and a 92% specificity for both entities (54,55). PSPW index was also significantly lower in refractory esophagitis, healed reflux esophagitis, and non-erosive esophagitis compared to functional heartburn. In another study, mean PSPW index was lower in non-erosive reflux disease (30%) compared to hypersensitive esophagus (51%), and functional heartburn (76%), and PSPW index was an independent predictor of hypersensitive esophagus [adjusted odds ratio (OR) =0.863, P=0.001) along with MNBI (adjusted OR =0.0998, P=0.001). PSPW index and MNBI were also able to differentiate 92% of patients with hypersensitive esophagus from functional heartburn compared to only 62% accurate differentiation by symptom association indexes (P<0.0001) alone (59). Finally, PSPW index, similar to MNBI (separately and combined) is able to identify PPI-responsive heartburn from non-responders better than AET (64). Given these parameters, PSPW Index can be a valuable tool in differentiating true reflux disease as a source of patient symptoms hypersensitivity or functional issues.
Wireless pH study (BRAVO®)
The wireless pH capsule study is another method for reflux testing; this does not require trans-nasal catheter placement. Also known as the Bravo® capsule, this device is a radiotelemetry capsule fitted with an antimony pH electrode that is temporarily affixed to the esophageal wall during a standard upper endoscopy (65). Patients are instructed to stop taking PPIs for at least 7 days prior to the study (38). They are asked if they are allergic to metals, especially nickel. The capsule transmits data to an external receiver by radiofrequency telemetry during the study period and subsequently self-detaches from the esophagus after 5–7 days (35). Patients log meal timing and symptom occurrence as they do with MII-pH testing. Symptoms and meal episodes logging instructions are similar to MII-pH study and probably more important for accurate AET and symptom association assessment as there is no concomitant impedance recording (Figure 9).
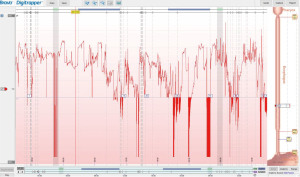
Compared to 24 hours of data with MII-pH, the wireless pH capsule provides monitoring for 48–96 hours, resulting in increased diagnostic yield and reproducibility (36,38,66), as there can be day-to-day variation. Diagnostic sensitivity is increased when only “worse day” AET is considered, albeit the specificity of the total AET is higher (32). This prolonged study time can help accurately diagnose patients with true reflux that only occurs infrequently and thus may be missed by 24-hour MII-pH. It also allows for patient stratification into different phenotypes which in turn can help guide treatment decisions. Hasak et al. described these phenotypes based on patterns observed on multi-day wireless pH studies (67). Patterns were called “concordant” when same acid exposure was observed among all 4 days, “dominant” when the same pattern was present on 2 or more days, and “discordant” for all other forms. In patients with 3 or more days of data available, 90.4% of the patients had a predominant pattern.
Wireless pH studies are less restricting and less uncomfortable compared to MII-pH and as a result patients are more likely to continue their normal daily activities during the study period, greatly increasing the tests utility (35). Wireless pH study appears to be relatively safe. Complications associated with wireless pH study are premature capsule detachment; this is recognized in the tracing as a drop in the pH to a prolonged acidic recording often to pH value of 2 followed by rapid increase in the pH to approximately 7 for the remainder of the study. Patients can have dysphagia, chest pain from the capsule; rarely, one has to endoscopically remove the capsule attached to the esophagus. The need for endoscopic detachment of the capsule appears to occur more frequently in patients with hypersensitive esophagus (65). In one study, 57 asymptomatic subjects underwent upper endoscopy and pH capsule placement. Esophagitis was seen in 6 cases and capsule dysfunction in 1 case (68). Capsule retention can rarely happen. Device-related adverse event report by the US Food and Drug Administration (FDA) indicates that since 2009, 30 cases of capsule retention were reported (69).
One often-cited negative factor for wireless pH capsule is its cost, however a decision model developed in one study comparing GERD management with and without wireless capsule use showed only a modest overall financial budgetary impact even with a 10% increase of utilization of the wireless pH capsule (70).
Summary
The GI motility laboratory continues to evolve and improve its ability to diagnosis esophageal disorders, both esophageal motility disorders and GERD. Through novel applications of older testing modalities as well as those that have been newly developed, more thorough evaluations of esophageal dysmotility and GERD are performed now than before. As these new tools become more refined and supported by a larger body of data, this trend should continue and frustrating gaps in knowledge regarding management of esophageal dysfunction will continue to close.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Abbas E. Abbas and Roman V. Petrov) for the series “New Technologies in Esophageal Surgery and Endoscopy” published in Annals of Esophagus. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-36/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-36/coif). The series “New Technologies in Esophageal Surgery and Endoscopy” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gyawali CP, Carlson DA, Chen JW, et al. ACG Clinical Guidelines: Clinical Use of Esophageal Physiologic Testing. Am J Gastroenterol 2020;115:1412-28. [Crossref] [PubMed]
- Yadlapati R. High Resolution Manometry Vs Conventional Line Tracing for Esophageal Motility Disorders. Gastroenterol Hepatol (N Y) 2017;13:176-8. [PubMed]
- Tack J, Pandolfino JE. Pathophysiology of Gastroesophageal Reflux Disease. Gastroenterology 2018;154:277-88. [Crossref] [PubMed]
- Ribolsi M, Savarino E, Rogers B, et al. High-resolution Manometry Determinants of Refractoriness of Reflux Symptoms to Proton Pump Inhibitor Therapy. J Neurogastroenterol Motil 2020;26:447-54. [Crossref] [PubMed]
- Yadlapati R, Kahrilas PJ, Fox MR, et al. Esophageal motility disorders on high-resolution manometry: Chicago classification version 4.0©. Neurogastroenterol Motil 2021;33:e14058. [Crossref] [PubMed]
- Wang D, Patel A, Mello M, et al. Esophagogastric junction contractile integral (EGJ-CI) quantifies changes in EGJ barrier function with surgical intervention. Neurogastroenterol Motil 2016;28:639-46. [Crossref] [PubMed]
- Gor P, Li Y, Munigala S, et al. Interrogation of esophagogastric junction barrier function using the esophagogastric junction contractile integral: an observational cohort study. Dis Esophagus 2016;29:820-8. [Crossref] [PubMed]
- Kataria R, Rosenfeld B, Malik Z, et al. Distal Esophageal Impedance Measured by High-resolution Esophageal Manometry With Impedance Suggests the Presence of Barrett's Esophagus. J Neurogastroenterol Motil 2020;26:344-51. [Crossref] [PubMed]
- Tobey NA, Argote CM, Vanegas XC, et al. Electrical parameters and ion species for active transport in human esophageal stratified squamous epithelium and Barrett's specialized columnar epithelium. Am J Physiol Gastrointest Liver Physiol 2007;293:G264-70. [Crossref] [PubMed]
- Caviglia R, Ribolsi M, Gentile M, et al. Dilated intercellular spaces and acid reflux at the distal and proximal oesophagus in patients with non-erosive gastro-oesophageal reflux disease. Aliment Pharmacol Ther 2007;25:629-36. [Crossref] [PubMed]
- Orlando LA, Orlando RC. Dilated intercellular spaces as a marker of GERD. Curr Gastroenterol Rep 2009;11:190-4. [Crossref] [PubMed]
- Horton A, Sullivan B, Charles K, et al. Esophageal Baseline Impedance From High-resolution Impedance Manometry Correlates With Mean Nocturnal Baseline Impedance From pH-impedance Monitoring. J Neurogastroenterol Motil 2020;26:455-62. [Crossref] [PubMed]
- Patel A, Wang D, Sainani N, et al. Distal mean nocturnal baseline impedance on pH-impedance monitoring predicts reflux burden and symptomatic outcome in gastro-oesophageal reflux disease. Aliment Pharmacol Ther 2016;44:890-8. [Crossref] [PubMed]
- Gyawali CP, Kahrilas PJ, Savarino E, et al. Modern diagnosis of GERD: the Lyon Consensus. Gut 2018;67:1351-62. [Crossref] [PubMed]
- Leopold A, Yu D, Bhuta R, et al. Multiple Rapid Swallows (MRS) Complements Single-Swallow (SS) Analysis for High-Resolution Esophageal Manometry (HREM). Dig Dis Sci 2019;64:2206-13. [Crossref] [PubMed]
- Shaker A, Stoikes N, Drapekin J, et al. Multiple rapid swallow responses during esophageal high-resolution manometry reflect esophageal body peristaltic reserve. Am J Gastroenterol 2013;108:1706-12. [Crossref] [PubMed]
- Woodland P, Gabieta-Sonmez S, Arguero J, et al. 200 mL Rapid Drink Challenge During High-resolution Manometry Best Predicts Objective Esophagogastric Junction Obstruction and Correlates With Symptom Severity. J Neurogastroenterol Motil 2018;24:410-4. [Crossref] [PubMed]
- Triggs JR, Carlson DA, Beveridge C, et al. Functional Luminal Imaging Probe Panometry Identifies Achalasia-Type Esophagogastric Junction Outflow Obstruction. Clin Gastroenterol Hepatol 2020;18:2209-17. [Crossref] [PubMed]
- Donnan EN, Pandolfino JE. EndoFLIP in the Esophagus: Assessing Sphincter Function, Wall Stiffness, and Motility to Guide Treatment. Gastroenterol Clin North Am 2020;49:427-35. [Crossref] [PubMed]
- Desprez C, Roman S, Leroi AM, et al. The use of impedance planimetry (Endoscopic Functional Lumen Imaging Probe, EndoFLIP®) in the gastrointestinal tract: A systematic review. Neurogastroenterol Motil 2020;32:e13980. [Crossref] [PubMed]
- Carlson DA, Kou W, Lin Z, et al. Normal Values of Esophageal Distensibility and Distension-Induced Contractility Measured by Functional Luminal Imaging Probe Panometry. Clin Gastroenterol Hepatol 2019;17:674-681.e1. [Crossref] [PubMed]
- Carlson DA, Gyawali CP, Kahrilas PJ, et al. Esophageal motility classification can be established at the time of endoscopy: a study evaluating real-time functional luminal imaging probe panometry. Gastrointest Endosc 2019;90:915-923.e1. [Crossref] [PubMed]
- Carlson DA, Kahrilas PJ, Lin Z, et al. Evaluation of Esophageal Motility Utilizing the Functional Lumen Imaging Probe. Am J Gastroenterol 2016;111:1726-35. [Crossref] [PubMed]
- Ngamruengphong S, von Rahden BH, Filser J, et al. Intraoperative measurement of esophagogastric junction cross-sectional area by impedance planimetry correlates with clinical outcomes of peroral endoscopic myotomy for achalasia: a multicenter study. Surg Endosc 2016;30:2886-94. [Crossref] [PubMed]
- Wu PI, Szczesniak MM, Craig PI, et al. Novel Intra-Procedural Distensibility Measurement Accurately Predicts Immediate Outcome of Pneumatic Dilatation for Idiopathic Achalasia. Am J Gastroenterol 2018;113:205-12. [Crossref] [PubMed]
- Nicodème F, Hirano I, Chen J, et al. Esophageal distensibility as a measure of disease severity in patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol 2013;11:1101-1107.e1. [Crossref] [PubMed]
- Naik RD, Evers L, Vaezi MF. Advances in the Diagnosis and Treatment of GERD: New Tricks for an Old Disease. Curr Treat Options Gastroenterol 2019;17:1-17. [Crossref] [PubMed]
- Kessing BF, Conchillo JM, Bredenoord AJ, et al. Review article: the clinical relevance of transient lower oesophageal sphincter relaxations in gastro-oesophageal reflux disease. Aliment Pharmacol Ther 2011;33:650-61. [Crossref] [PubMed]
- Bytzer P, Jones R, Vakil N, et al. Limited ability of the proton-pump inhibitor test to identify patients with gastroesophageal reflux disease. Clin Gastroenterol Hepatol 2012;10:1360-6. [Crossref] [PubMed]
- Richter JE, Rubenstein JH. Presentation and Epidemiology of Gastroesophageal Reflux Disease. Gastroenterology 2018;154:267-76. [Crossref] [PubMed]
- Dent J, Vakil N, Jones R, et al. Accuracy of the diagnosis of GORD by questionnaire, physicians and a trial of proton pump inhibitor treatment: the Diamond Study. Gut 2010;59:714-21. [Crossref] [PubMed]
- Ang D, Lee YY, Clarke JO, et al. Diagnosis of gastroesophageal reflux: an update on current and emerging modalities. Ann N Y Acad Sci 2020;1481:154-69. [Crossref] [PubMed]
- Dent J, Becher A, Sung J, et al. Systematic review: patterns of reflux-induced symptoms and esophageal endoscopic findings in large-scale surveys. Clin Gastroenterol Hepatol 2012;10:863-873.e3. [Crossref] [PubMed]
- Kahrilas PJ, Quigley EM. Clinical esophageal pH recording: a technical review for practice guideline development. Gastroenterology 1996;110:1982-96. [Crossref] [PubMed]
- Kamal AN, Clarke JO, Oors JM, et al. The Role of Symptom Association Analysis in Gastroesophageal Reflux Testing. Am J Gastroenterol 2020;115:1950-9. [Crossref] [PubMed]
- Savarino E, Bredenoord AJ, Fox M, et al. Expert consensus document: Advances in the physiological assessment and diagnosis of GERD. Nat Rev Gastroenterol Hepatol 2017;14:665-76. [Crossref] [PubMed]
- Ravi K, Katzka DA. Esophageal Impedance Monitoring: Clinical Pearls and Pitfalls. Am J Gastroenterol 2016;111:1245-56. [Crossref] [PubMed]
- Mattox HE 3rd, Richter JE, Sinclair JW, et al. Gastroesophageal pH step-up inaccurately locates proximal border of lower esophageal sphincter. Dig Dis Sci 1992;37:1185-91. [Crossref] [PubMed]
- Klauser AG, Schindlbeck NE, Müller-Lissner SA. Esophageal 24-h pH monitoring: is prior manometry necessary for correct positioning of the electrode? Am J Gastroenterol 1990;85:1463-7. [PubMed]
- Wiener GJ, Morgan TM, Copper JB, et al. Ambulatory 24-hour esophageal pH monitoring. Reproducibility and variability of pH parameters. Dig Dis Sci 1988;33:1127-33. [Crossref] [PubMed]
- Patel A, Sayuk GS, Gyawali CP. Parameters on esophageal pH-impedance monitoring that predict outcomes of patients with gastroesophageal reflux disease. Clin Gastroenterol Hepatol 2015;13:884-91. [Crossref] [PubMed]
- Mittal RK, Holloway RH, Penagini R, et al. Transient lower esophageal sphincter relaxation. Gastroenterology 1995;109:601-10. [Crossref] [PubMed]
- Vela MF, Camacho-Lobato L, Srinivasan R, et al. Simultaneous intraesophageal impedance and pH measurement of acid and nonacid gastroesophageal reflux: effect of omeprazole. Gastroenterology 2001;120:1599-606. [Crossref] [PubMed]
- Tutuian R, Castell DO. Review article: complete gastro-oesophageal reflux monitoring - combined pH and impedance. Aliment Pharmacol Ther 2006;24:27-37. [Crossref] [PubMed]
- Gyawali CP, Rogers B, Frazzoni M, et al. Inter-reviewer Variability in Interpretation of pH-Impedance Studies: The Wingate Consensus. Clin Gastroenterol Hepatol 2021;19:1976-8.e1. [Crossref] [PubMed]
- Pandolfino JE, Richter JE, Ours T, et al. Ambulatory esophageal pH monitoring using a wireless system. Am J Gastroenterol 2003;98:740-9. [Crossref] [PubMed]
- Penagini R, Sweis R, Mauro A, et al. Inconsistency in the Diagnosis of Functional Heartburn: Usefulness of Prolonged Wireless pH Monitoring in Patients With Proton Pump Inhibitor Refractory Gastroesophageal Reflux Disease. J Neurogastroenterol Motil 2015;21:265-72. [Crossref] [PubMed]
- Prakash C, Jonnalagadda S. Esophageal impedance testing: unraveling the mysteries of gastroesophageal reflux. Gastroenterology 2006;131:322-3. [Crossref] [PubMed]
- Loots CM, van Wijk MP, Blondeau K, et al. Interobserver and intraobserver variability in pH-impedance analysis between 10 experts and automated analysis. J Pediatr 2012;160:441-446.e1. [Crossref] [PubMed]
- Roman S, Bruley des Varannes S, Pouderoux P, et al. Ambulatory 24-h oesophageal impedance-pH recordings: reliability of automatic analysis for gastro-oesophageal reflux assessment. Neurogastroenterol Motil 2006;18:978-86. [Crossref] [PubMed]
- Bredenoord AJ, Weusten BL, Smout AJ. Symptom association analysis in ambulatory gastro-oesophageal reflux monitoring. Gut 2005;54:1810-7. [Crossref] [PubMed]
- Weusten BL, Roelofs JM, Akkermans LM, et al. The symptom-association probability: an improved method for symptom analysis of 24-hour esophageal pH data. Gastroenterology 1994;107:1741-5. [Crossref] [PubMed]
- Kushnir VM, Sathyamurthy A, Drapekin J, et al. Assessment of concordance of symptom reflux association tests in ambulatory pH monitoring. Aliment Pharmacol Ther 2012;35:1080-7. [Crossref] [PubMed]
- Frazzoni M, Savarino E, de Bortoli N, et al. Analyses of the Post-reflux Swallow-induced Peristaltic Wave Index and Nocturnal Baseline Impedance Parameters Increase the Diagnostic Yield of Impedance-pH Monitoring of Patients With Reflux Disease. Clin Gastroenterol Hepatol 2016;14:40-6. [Crossref] [PubMed]
- Frazzoni M, de Bortoli N, Frazzoni L, et al. The added diagnostic value of postreflux swallow-induced peristaltic wave index and nocturnal baseline impedance in refractory reflux disease studied with on-therapy impedance-pH monitoring. Neurogastroenterol Motil 2017; [Crossref] [PubMed]
- Rengarajan A, Savarino E, Della Coletta M, et al. Mean Nocturnal Baseline Impedance Correlates With Symptom Outcome When Acid Exposure Time Is Inconclusive on Esophageal Reflux Monitoring. Clin Gastroenterol Hepatol 2020;18:589-95. [Crossref] [PubMed]
- Farré R, Blondeau K, Clement D, et al. Evaluation of oesophageal mucosa integrity by the intraluminal impedance technique. Gut 2011;60:885-92. [Crossref] [PubMed]
- Martinucci I, de Bortoli N, Savarino E, et al. Esophageal baseline impedance levels in patients with pathophysiological characteristics of functional heartburn. Neurogastroenterol Motil 2014;26:546-55. [Crossref] [PubMed]
- Frazzoni M, de Bortoli N, Frazzoni L, et al. Impairment of chemical clearance and mucosal integrity distinguishes hypersensitive esophagus from functional heartburn. J Gastroenterol 2017;52:444-51. [Crossref] [PubMed]
- Kandulski A, Weigt J, Caro C, et al. Esophageal intraluminal baseline impedance differentiates gastroesophageal reflux disease from functional heartburn. Clin Gastroenterol Hepatol 2015;13:1075-81. [Crossref] [PubMed]
- Frazzoni M, Manta R, Mirante VG, et al. Esophageal chemical clearance is impaired in gastro-esophageal reflux disease--a 24-h impedance-pH monitoring assessment. Neurogastroenterol Motil 2013;25:399-406, e295.
- de Bortoli N, Martinucci I, Savarino E, et al. Association between baseline impedance values and response proton pump inhibitors in patients with heartburn. Clin Gastroenterol Hepatol 2015;13:1082-8.e1. [Crossref] [PubMed]
- Frazzoni M, Bertani H, Manta R, et al. Impairment of chemical clearance is relevant to the pathogenesis of refractory reflux oesophagitis. Dig Liver Dis 2014;46:596-602. [Crossref] [PubMed]
- Frazzoni L, Frazzoni M, de Bortoli N, et al. Postreflux swallow-induced peristaltic wave index and nocturnal baseline impedance can link PPI-responsive heartburn to reflux better than acid exposure time. Neurogastroenterol Motil 2017; [Crossref] [PubMed]
- Aulakh S, Ashley S, Haas K, et al. Esophageal pH Capsule Retention. ACG Case Rep J 2020;7:e00383. [Crossref] [PubMed]
- Scarpulla G, Camilleri S, Galante P, et al. The impact of prolonged pH measurements on the diagnosis of gastroesophageal reflux disease: 4-day wireless pH studies. Am J Gastroenterol 2007;102:2642-7. [Crossref] [PubMed]
- Hasak S, Yadlapati R, Altayar O, et al. Prolonged Wireless pH Monitoring in Patients With Persistent Reflux Symptoms Despite Proton Pump Inhibitor Therapy. Clin Gastroenterol Hepatol 2020;18:2912-9. [Crossref] [PubMed]
- Wenner J, Johnsson F, Johansson J, et al. Wireless oesophageal pH monitoring: feasibility, safety and normal values in healthy subjects. Scand J Gastroenterol 2005;40:768-74. [Crossref] [PubMed]
- MAUDE - Manufacturer and User Facility Device Experience. Accessed September 13 2019.9. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfMAUDE/search.CFM
- Lee WC, Yeh YC, Lacy BE, et al. Timely confirmation of gastro-esophageal reflux disease via pH monitoring: estimating budget impact on managed care organizations. Curr Med Res Opin 2008;24:1317-27. [Crossref] [PubMed]
Cite this article as: Malamood M, Shahsavari D, Parkman HP. Modern evaluation of esophageal function in the gastrointestinal motility laboratory: a narrative review. Ann Esophagus 2023;6:19.


