Considerations in the management of gastroesophageal reflux disease in the morbidly obese
Introduction
Gastroesophageal reflux disease (GERD) is a common problem worldwide. The prevalence of GERD related symptoms found in the obese and morbidly obese population have been demonstrated to be consistently higher than the non-obese patients. There are many publications demonstrating a positive correlation of increased BMI and reflux related symptoms (38–39% in patient withs BMI >30 kg/m2) or reflux demonstrated on pH monitoring (1,2). Here we provide a brief review and common treatment pathways to deal with GERD in the obese population.
Pathophysiology of GERD in obese patients
The pathophysiology of GERD in obese patients is multifactorial and must be understood to fully treat this problem. It can be thought of as two entities; one is a mechanical/anatomic failure of the body’s natural anti-reflux mechanisms and the other is biochemical changes related to obesity.
Anatomic/mechanical deficiencies
- The lower esophageal sphincter (LES) acts to create a high-pressure zone just proximal to the esophagogastric junction (EGJ) in order to oppose the thoracoabdominal pressure gradient thus preventing the reflux of gastric contents into the thoracic esophagus (3). A defective LES may be a sequalae of a hiatal hernia (HH) which is common entity of the obese patient (nearly 40% HH found in morbidly obese patients) (4). HH may lead to hypotensive LES thus allowing intragastric pressure to overcome the LES and cause reflux into the chest. Transient LES relaxations (TLESRs) are spontaneous LES relaxations not triggered by swallowing mechanisms and are thought to be initiated by a vasovagal reflex. TLESRs may be associated with gastric distention and overeating and have been found to be more frequent in obese individuals leading to high rates of GERD (5-7).
- Increased intra-abdominal pressure secondary to increased intra-abdominal adiposity provides an altered thoracoabdominal pressure gradient favoring GERD in the obese population. This also leads to an upward displacement of the EGJ into the chest thus decreasing the diaphragm’s impact on the LES and promoting GERD symptoms (8).
- There are conflicting reports as to whether there is decreased esophageal and gastric motility leading to reflux in obese patients, but these could be an important component to this complex disease process (9,10).
Biochemical alterations
- Diet in obese patients may be different from that of non-obese patients. Increased fatty food intake has been linked to a reduction in endogenous and exogenous effects of gastrin thus leading to a decrease in LES pressures and postprandial reflux. High carbohydrate intake exposes the colon and TI to increased levels of short-chain fatty acids which triggers proximal stomach relaxation and increased TLESRs and reflux (11,12).
- Hormones such as Ghrelin and Leptin may be important mechanisms for GERD development in the obese population as the can affect gastric motility (Ghrelin) and LES tone (Leptin) (13,14). Decreased levels of adiponectin may be linked to GERD/erosive esophagitis in obese patients and further investigation is warranted (15).
Operations in the obese population for GERD
Fundoplication
Laparoscopic anti-reflux operations such as the Nissen fundoplication are safe and effective operations for the treatment of GERD. The safety profile in obese patients also seems acceptable (16). There has been conflicting evidence on the efficacy of fundoplication for GERD in the obese patient. Several publications have demonstrated comparable outcomes in terms of GERD relief after fundoplication in obese and non-obese patients, but long-term efficacy was still in question (17-19). A recent meta-analysis looked at multiple studies comparing obese (BMI >30) and non-obese patients (BMI <30) undergoing fundoplication for GERD and showed that when the data was collectively pooled, a statistically significant increase in recurrence by 53% (RR =1.53) was noted in the obese population (20). Although fundoplication in not contraindicated in the obese population, these patients should be counseled on the benefits of bariatric surgery which can treat not only the patient’s reflux symptoms but also provide added benefits of weight loss and comorbidity resolution. Fundoplication may still be used in the obese population in circumstances that prohibit bariatric surgery (anatomic, socioeconomic and/or insurance coverage).
Sleeve gastrectomy (SG)
SG has become the most performed bariatric operation in the world today. There have been several publications demonstrating conflicting results when considering GERD and SG. Stenard and Iannelli conducted the largest systematic review concerning SG and GERD which included 25 studies. Thirteen studies concluded worsening symptoms of GERD and twelve demonstrated clinical improvement of GERD (21). Oor et al. conducted a meta-analysis including 33 studies and 8,092 patients undergoing SG which demonstrated the incidence of de novo GERD to be 20% (22). Genco et al. demonstrated a development of Barrett’s Esophagus (BE) in post SG patients undergoing surveillance endoscopy in 17.2% of patients that of which 26.4% did not have symptoms of GERD, thus concerning for asymptomatic reflux in SG patients (23). In an analysis of the Bariatric Outcomes Longitudinal Database (BOLD), Dupree et al. had shown 44.5% of patients undergoing SG had symptomatic GERD preoperatively. Of these patients, 84.1% had continued symptoms, 9% had worsening symptoms, and only 15.9% had resolution of symptoms. They also demonstrated an 8.6% incidence of de novo GERD symptoms (24). There are several factors that may lead to the development or worsening of GERD post SG. These include a missed HH, increased gastric pressure from a narrowing of gastric lumen, blunting of the angle of His, retained fundus or decreased LES tone from division of the oblique sling fibers. Evidence supports routine repair of any HH with SG and some authors have advocated for fundoplication at the time of surgery with good results in small case series. SG is relatively contraindicated as the bariatric procedure in patients with preoperative GERD. Sleeve may, however, be performed if there is a contraindication to gastric bypass or because of patient preference after adequate counselling. The authors recommend gastric bypass in patients with significant reflux symptoms and if SG is considered we would recommend investigating esophageal motility prior to sleeve.
Roux-en-Y Gastric Bypass (RYGB)
The RYGB is currently considered the gold standard operation for patients with morbid obesity and GERD. The mechanisms by which the RYGB treats and prevents GERD are multifactorial and include a small gastric pouch (minimal parietal cell mass and acid production), low pressure gradient over LES, diversion of bile from Roux limb, and weight loss without disruption of the natural anti-reflux mechanisms (25). Multiple studies have shown the undeniable efficacy of the RYGB in terms of typical/atypical GERD symptom relief, antisecretory medication use, and DeMeester score. Symptom relief and medication cessation have been seen in up to 94% in some series after a RYGB (26-29). Another consideration of recent interest has been patients who have Barrett’s Esophagus (BE). BE is more prevalent with obesity and its risk increases with each BMI point (30). There are several publications demonstrating the efficacy of RYGB in patients who have BE, showing regression of BE in 40% and halting of progression in nearly all patients (31-36). It is our practice to offer all patients with documented BE, Erosive Esophagitis (EE) and significant GERD a RYGB if no contraindications are present. An additional subset of patients that would benefit from RYGB are those with prior failed fundoplication. A recent meta-analysis evaluating 118 such patients (mean BMI of 37.17 kg/m2) demonstrated total remission of symptoms in 89.8% and at least partial remission in the remaining 10.2%. These operations did have an overall higher yet acceptable rate of complications when compared to primary RYGB (37). Further studies are warranted to recommend RYGB for refractory GERD after failed fundoplication in non-obese patients, however this approach is this authors’ preferred algorithm and a retrospective study by our institution is underway. Controversy surrounds the recommendation for a RYGB in GERD patients with a BMI of 30–35 kg/m2 as a primary operation over fundoplication; however, this option is difficult in the United States as most insurance companies have specific requirements for a BMI above 35 kg/m2. Conversion to a RYGB for a patient with refractory GERD following a prior SG has been shown to be quite effective and is our treatment of choice in these patients (38,39).
It is the author’s opinion and preference to offer RYGB to obese patient’s presenting with GERD symptoms. If there is any HH present, then repair is also offered at the time of surgery. It is our belief that SG in the setting of GERD is a relative contraindication but can be considered in certain situations. Our technique of RYGB does not differ when considering the bariatric patient with or without GERD preoperatively. Care is taken to ensure they have a sufficiently long roux limb to avoid any future bile reflux. Thus far, we have been able to achieve considerable reduction and/or resolution of GERD symptoms in obese patients undergoing RYGB. With this selective technique, we have seen a very small percentage of patients requiring revisional surgery of their sleeve to a bypass for GERD.
Technique
Our preferred technique of minimally invasive gastric bypass is done laparoscopically as depicted below:
- Access and exposure
- Patient position supine with straps and footboard
- Operating surgeon on patient’s right side and assistant on left
- Veress needle pneumo-insufflation in LUQ to 15 mmHg
- 5 mm optical trocar entrance
- Steep reverse Trendelenburg
- Port placement:
- 5 mm right subcostal midaxillary line—flexible liver retractor
- 5 mm right subcostal anterior axillary line—surgeon left hand
- 12 mm right midabdominal—surgeon right hand
- 5 mm left midabdominal—5 mm 30-degree laparoscope
- 5 mm left subcostal midaxillary line—assistant left hand
- 5 mm left subcostal anterior axillary line—assistant right hand
- Pouch creation:
- Mobilize angle of His
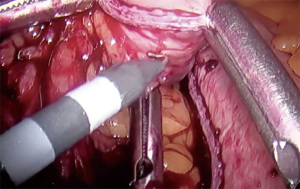
- Perigastric dissection between the first and second lesser curvature vessels to create a retrogastric tunnel (Figure 1)
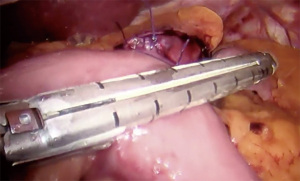
- 45 mm medium staple height sized stapler used to transect stomach from lesser curve towards greater curve
- Completion of retrogastric dissection to angle of His and to expose left crus
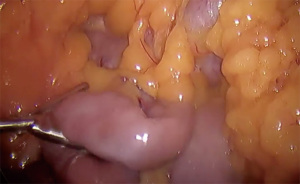
- Sequential firing of 60 mm stapler directed cranially to create pouch approximately 15–30 mL in size (Figure 2)
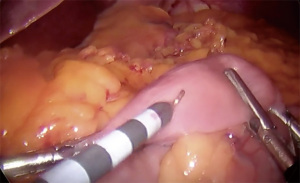
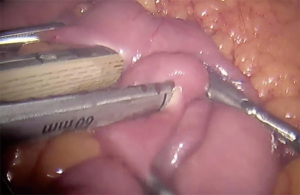
- Creation of gastrotomy utilizing hook electrocautery (cutting function) on posterior pouch (Figure 3)
- Creation of omega loop gastrojejunostomy
- Divide omentum
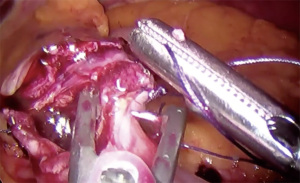
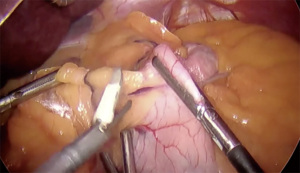
- Retract transverse colon cephalad and anteriorly to expose the ligament of Treitz (Figure 4)
- Measure 60 cm distally from ligament of Treitz [biliopancreatic (BP) limb] careful to keep orientation of bowel without twisting
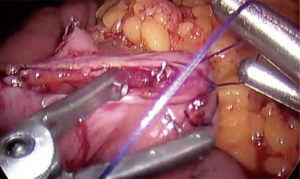
- Creation of jejunotomy utilizing hook electrocautery (cutting function) (Figure 5)
- Creation of 2.5 cm gastrojejunostomy with posterior gastric pouch to antimesenteric jejunum with linear cutting stapler (Figure 6)
- Closure of common enterotomy with running 2-0 absorbable suture (Figure 7)
- Creation of jejunojejunostomy
- Division of BP limb from gastrojejunal (GJ) anastomosis (Figure 8)
- Measure 150 cm distally from GJ anastomosis on Roux limb
- Creation of jejunotomy in Roux limb and BP limb utilizing hook electrocautery (cutting function)
- Creation of jejunojejunostomy with a 60 mm short staples linear cutting stapler (Figure 9) and closure of common enterotomy with running 2-0 absorbable suture (Figure 10)
- Closure of mesenteric defects
- Jejunojejunostomy mesenteric defect closure with running non absorbable suture
- Peterson’s mesenteric defect closure with running non absorbable suture
- Intraoperative endoscopy and GJ leak test (air insufflated under saline)
- Closure of ports
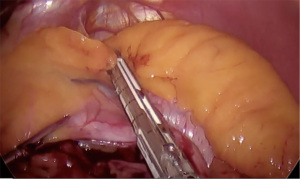
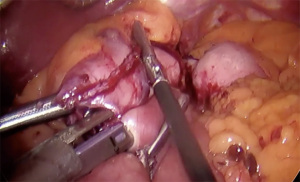
Post-operatively, patients are routinely discharged on POD 1 without any imaging on a bariatric full liquid diet for 3 weeks. They are discharged with limited pain medication, stool softeners, anti-nausea medications, a multivitamin as well as a PPI. We see them at 3 weeks, 3 months, 6 months and then every 6 months post-operatively.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Timothy M. Farrell and Geoffrey Kohn) for the series “Minimally Invasive Procedures for Gastroesophageal Reflux Disease” published in Annals of Esophagus. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-20/coif). The series “Minimally Invasive Procedures for Gastroesophageal Reflux Disease” was commissioned by the editorial office without any funding or sponsorship. ADP has received payments for speaking from Medtronic, Gore, Stryker, Merck and Ethicon and has provided expert opinion to Medtronic. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hong D, Khajanchee YS, Pereira N, et al. Manometric abnormalities and gastroesophageal reflux disease in the morbidly obese. Obes Surg 2004;14:744-9. [Crossref] [PubMed]
- Dent J, El-Serag HB, Wallander MA, et al. Epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut 2005;54:710-7. [Crossref] [PubMed]
- Patti MG, Gantert W, Way LW. Surgery of the esophagus. Anatomy and physiology. Surg Clin North Am 1997;77:959-70. [Crossref] [PubMed]
- Che F, Nguyen B, Cohen A, et al. Prevalence of hiatal hernia in the morbidly obese. Surg Obes Relat Dis 2013;9:920-4. [Crossref] [PubMed]
- Wu JC, Mui LM, Cheung CM, et al. Obesity is associated with increased transient lower esophageal sphincter relaxation. Gastroenterology 2007;132:883-9. [Crossref] [PubMed]
- Schneider JH, Küper M, Königsrainer A, et al. Transient lower esophageal sphincter relaxation in morbid obesity. Obes Surg 2009;19:595-600. [Crossref] [PubMed]
- Ayazi S, Tamhankar A, DeMeester SR, et al. The impact of gastric distension on the lower esophageal sphincter and its exposure to acid gastric juice. Ann Surg 2010;252:57-62. [Crossref] [PubMed]
- Pandolfino JE, El-Serag HB, Zhang Q, et al. Obesity: a challenge to esophagogastric junction integrity. Gastroenterology 2006;130:639-49. [Crossref] [PubMed]
- Küper MA, Kramer KM, Kirschniak A, et al. Dysfunction of the lower esophageal sphincter and dysmotility of the tubular esophagus in morbidly obese patients. Obes Surg 2009;19:1143-9. [Crossref] [PubMed]
- Mushref MA, Srinivasan S. Effect of high fat-diet and obesity on gastrointestinal motility. Ann Transl Med 2013;1:14. [PubMed]
- Pehl C, Pfeiffer A, Waizenhoefer A, et al. Effect of caloric density of a meal on lower oesophageal sphincter motility and gastro-oesophageal reflux in healthy subjects. Aliment Pharmacol Ther 2001;15:233-9. [Crossref] [PubMed]
- Piche T, des Varannes SB, Sacher-Huvelin S, et al. Colonic fermentation influences lower esophageal sphincter function in gastroesophageal reflux disease. Gastroenterology 2003;124:894-902. [Crossref] [PubMed]
- Myers MG, Cowley MA, Münzberg H. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol 2008;70:537-56. [Crossref] [PubMed]
- Nahata M, Saegusa Y, Harada Y, et al. Changes in ghrelin-related factors in gastroesophageal reflux disease in rats. Gastroenterol Res Pract 2013;2013:504816. [Crossref] [PubMed]
- Kato M, Watabe K, Hamasaki T, et al. Association of low serum adiponectin levels with erosive esophagitis in men: an analysis of 2405 subjects undergoing physical check-ups. J Gastroenterol 2011;46:1361-7. [Crossref] [PubMed]
- Telem DA, Altieri M, Gracia G, et al. Perioperative outcome of esophageal fundoplication for gastroesophageal reflux disease in obese and morbidly obese patients. Am J Surg 2014;208:163-8. [Crossref] [PubMed]
- Anvari M, Bamehriz F. Outcome of laparoscopic Nissen fundoplication in patients with body mass index >or=35. Surg Endosc 2006;20:230-4. [Crossref] [PubMed]
- D'Alessio MJ, Arnaoutakis D, Giarelli N, et al. Obesity is not a contraindication to laparoscopic Nissen fundoplication. J Gastrointest Surg 2005;9:949-54. [Crossref] [PubMed]
- Winslow ER, Frisella MM, Soper NJ, et al. Obesity does not adversely affect the outcome of laparoscopic antireflux surgery (LARS). Surg Endosc 2003;17:2003-11. [Crossref] [PubMed]
- Bashir Y, Chonchubhair HN, Duggan SN, et al. Systematic review and meta-analysis on the effect of obesity on recurrence after laparoscopic anti-reflux surgery. Surgeon 2019;17:107-18. [Crossref] [PubMed]
- Stenard F, Iannelli A. Laparoscopic sleeve gastrectomy and gastroesophageal reflux. World J Gastroenterol 2015;21:10348-57. [Crossref] [PubMed]
- Oor JE, Roks DJ, Ünlü Ç, et al. Laparoscopic sleeve gastrectomy and gastroesophageal reflux disease: a systematic review and meta-analysis. Am J Surg 2016;211:250-67. [Crossref] [PubMed]
- Genco A, Soricelli E, Casella G, et al. Gastroesophageal reflux disease and Barrett's esophagus after laparoscopic sleeve gastrectomy: a possible, underestimated long-term complication. Surg Obes Relat Dis 2017;13:568-74. [Crossref] [PubMed]
- DuPree CE, Blair K, Steele SR, et al. Laparoscopic sleeve gastrectomy in patients with preexisting gastroesophageal reflux disease: a national analysis. JAMA Surg 2014;149:328-34. [Crossref] [PubMed]
- Nadaleto BF, Herbella FA, Patti MG. Gastroesophageal reflux disease in the obese: Pathophysiology and treatment. Surgery 2016;159:475-86. [Crossref] [PubMed]
- Frezza EE, Ikramuddin S, Gourash W, et al. Symptomatic improvement in gastroesophageal reflux disease (GERD) following laparoscopic Roux-en-Y gastric bypass. Surg Endosc 2002;16:1027-31. [Crossref] [PubMed]
- Nelson LG, Gonzalez R, Haines K, et al. Amelioration of gastroesophageal reflux symptoms following Roux-en-Y gastric bypass for clinically significant obesity. Am Surg 2005;71:950-3; discussion 953-4. [Crossref] [PubMed]
- Madalosso CA, Gurski RR, Callegari-Jacques SM, et al. The Impact of Gastric Bypass on Gastroesophageal Reflux Disease in Morbidly Obese Patients. Ann Surg 2016;263:110-6. [Crossref] [PubMed]
- Mejía-Rivas MA, Herrera-López A, Hernández-Calleros J, et al. Gastroesophageal reflux disease in morbid obesity: the effect of Roux-en-Y gastric bypass. Obes Surg 2008;18:1217-24. [Crossref] [PubMed]
- Hofstetter WL, Peters JH, DeMeester TR, et al. Long-term outcome of antireflux surgery in patients with Barrett's esophagus. Ann Surg 2001;234:532-8; discussion 538-9. [Crossref] [PubMed]
- Houghton SG, Romero Y, Sarr MG. Effect of Roux-en-Y gastric bypass in obese patients with Barrett's esophagus: attempts to eliminate duodenogastric reflux. Surg Obes Relat Dis 2008;4:1-4; discussion 4-5. [Crossref] [PubMed]
- Gorodner V, Buxhoeveden R, Clemente G, et al. Barrett's esophagus after Roux-en-Y gastric bypass: does regression occur? Surg Endosc 2017;31:1849-54. [Crossref] [PubMed]
- Andrew B, Alley JB, Aguilar CE, et al. Barrett's esophagus before and after Roux-en-Y gastric bypass for severe obesity. Surg Endosc 2018;32:930-6. [Crossref] [PubMed]
- Csendes A, Burgos AM, Smok G, et al. Effect of gastric bypass on Barrett's esophagus and intestinal metaplasia of the cardia in patients with morbid obesity. J Gastrointest Surg 2006;10:259-64. [Crossref] [PubMed]
- Braghetto I, Korn O, Csendes A, et al. Laparoscopic treatment of obese patients with gastroesophageal reflux disease and Barrett's esophagus: a prospective study. Obes Surg 2012;22:764-72. [Crossref] [PubMed]
- Signorini F, Viscido G, Bocco MCA, et al. Impact of Gastric Bypass on Erosive Esophagitis and Barret's Esophagus. Obes Surg 2020;30:1194-9. [Crossref] [PubMed]
- Mendes-Filho AM, Godoy ESN, Alhinho HCAW, et al. Fundoplication conversion in Roux-En-Y gastric bypass for control of obesity and gastroesophageal reflux: systematic review. Arq Bras Cir Dig 2017;30:279-82. [Crossref] [PubMed]
- Amiki M, Seki Y, Kasama K, et al. Revisional Bariatric Surgery for Insufficient Weight Loss and Gastroesophageal Reflux Disease: Our 12-Year Experience. Obes Surg 2020;30:1671-8. [Crossref] [PubMed]
- Parmar CD, Mahawar KK, Boyle M, et al. Conversion of Sleeve Gastrectomy to Roux-en-Y Gastric Bypass is Effective for Gastro-Oesophageal Reflux Disease but not for Further Weight Loss. Obes Surg 2017;27:1651-8. [Crossref] [PubMed]
Cite this article as: Seeras K, Campbell J, Pryor AD. Considerations in the management of gastroesophageal reflux disease in the morbidly obese. Ann Esophagus 2022;5:41.