Endoscopic resection techniques of benign esophageal tumors: literature review
Introduction
Benign esophageal tumors (BET) are rare, with reported overall incidence rates of <1% in an autopsy series (1).Recent increases in access to affordable diagnostic tools have amplified their incidental finding rates and subsequently brought to the forefront important questions of their optimal management (2). More than 50% of benign esophageal masses are asymptomatic and they account for <10% of surgically resected esophageal tumors (3,4). Dysphagia is the most common symptom and typically correlates with larger tumor size. Additionally, as a consequence of the slow-growing nature of these lesions, patients typically present with chronic symptoms that have been reported to range from 11 months to >5 years (4).Other important symptoms include pain, heartburn, upper gastrointestinal (GI) bleeding, and in pediatric patients, respiratory symptoms.
BETs are histologically heterogeneous and can arise from any of the types of esophageal cells, layers, and at any level of the esophagus. There are multiple classification systems available; with the most surgically inclined system focusing on the extent of tissue invasion by the tumor. Thus, an important classification of BETs relates to the extent of tumor invasion: intraluminal, intramural, and extraesophageal affecting interventional planning (5).
Intraluminal tumors arise from the mucosal and submucosal layers, with fibrovascular polyps, lipomatous and epithelial polyps encompassing the majority of these lesions. These often result from an outgrowth of mucosal (squamous/columnar) cells or submucosal (glandular, vascular, and neural cells) layers with breach into the muscularis, adventitial layers, or the esophageal lumen. Other intraluminal tumors include adenomas, papillomas, hemangiomas, and granular cell tumors.
Intramural tumors include leiomyomas, gastrointestinal stromal tumors (GISTs), and Schwannomas. Leiomyomas are the most common BET, usually found in the middle to the distal third of the esophagus, and originate from the smooth muscle cells of the muscularis propria (MP). GISTs are located in the distal third of the esophagus and are thought to arise from the pacemaker cells of Cajal. GISTs have the highest malignant potential of all BETs. Esophageal schwannomas are the rarest BET, originate from Schwann cells in the neural plexus, and are often found in the upper third of the esophagus.
Extraesophageal BETs arise from the adventitial tissue and are usually malformations of the esophagus, and are often cysts and duplications of surrounding bronchogenic, neurenteric, and epithelial origin (5).
After appropriate history and physical exam, symptomatic patients typically undergo a contrast swallow study with these benign tumors presenting with a smooth contoured filling defect. An upper endoscopy allows for the direct visualization of the esophageal anatomy, tumor level, extent, and tissue acquisition. An endoscopic ultrasound (EUS) will provide appropriate localization of esophageal tumor based on the esophageal layer; an indispensable characterization for management planning (6). It provides important anatomic and diagnostic landmarks that guide surgical and endoscopic planning by delineating the layer of the esophageal tumor involvement, specifically, whether the tumor is extramural or intramural without the engagement of the superficial mucosal layer (7). Furthermore, the EUS will provide accurate lesion size measurements, will aid in determining the vascularity of the interrogated tumor, and can define the regularity of borders and cell layers involved (8). A computed tomography (CT) scan of the chest and upper abdomen helps delineate the location of the lesion and its relationship to the other vital structures in the mediastinum.
Important indications for surgical intervention of esophageal tumors are rapid tumor growth, morbid symptomatology, mucosal ulceration, and histologic diagnosis of potential for malignancy. On the other hand, small (<1 cm), asymptomatic non enlarging BET can be observed. This review article will describe important considerations for interventions on BET with a focus on endoscopic techniques. Important advancements of endoscopic technology and improvements in patient outcomes have driven the adoption of the below-discussed techniques of endoscopic resection of BETs (9). We present the following article in accordance with the Narrative Review reporting checklist (available at: https://aoe.amegroups.com/article/view/10.21037/aoe-21-32/rc).
Methods
For this review study, electronic databases were searched by two reviewers independently, including MEDLINE via PubMed, Cochrane Database, and Google Scholar for published studies in English language mapping to MeSH terms “endoscopic techniques”, “endoscopic mucosal resection”, “submucosal dissection”, “submucosal tunneling”, “full-thickness resection”, “endoscopic submucosal excavation”, “esophageal tumors” and “benign”. Articles that included tumor resections in areas in the GI tract apart from the esophagus were excluded. Only BET with the least aggressive potential were studied including Gastrointestinal Stromal tumors, Leiomyoma, Lipoma, Granular Cell tumor, Schwannomas, etc. Patients who underwent endoscopic resection of malignant or premalignant esophageal lesions including Barret’s esophagus were excluded from the study. The data from the published articles were recorded including procedural descriptions, study period, population number, tumor size, and location as well as procedural time and technique. Procedural success, as well as complications, tumor recurrence, and follow-up, were also compiled and reported. The technical success rate and post-resection tumor recurrence were reported in percentage while numeric variables including operative time and follow-up were reported with mean +/- standard deviation or median with range (min-max) per the reporting article. Missing data were reported as not available. Given heterogenicity among the studies, their retrospective nature, and small sample sizes, no comparative statistical analysis was performed.
Discussion/summary
Techniques
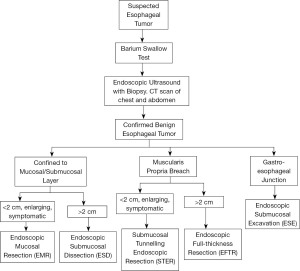
In the management of BETs, the breach of the MP is the most important factor in the choice of resection techniques (10).The techniques described below have different tissue acquisition potential, unique indications, and potential complications. Tumors located strictly in the submucosal layer are managed via endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD). On the other hand, tumors involving the MP undergo endoscopic full-thickness resection (EFTR) or submucosal tunneling endoscopic resection (STER). Based on the tumor size, location and extension, an algorithm can be used to help guide the surgical treatment of BETs (Figure 1).
Endoscopic mucosal resection (EMR)
EMR is basically a polypectomy technique. It can be done by inducing a submucosal layer separation from the MP using submucosal needle injections. The most common injection solutions include hypertonic saline, sodium hyaluronate, 50% dextrose, and a 4% succinate gelatin sometimes mixed with a coloring dye like methylene blue to allow for tissue differentiation (11). This technique creates a fluid barrier to “lift and cut” the BET and reduce the likelihood of perforating the MP layer. Resection and hemostasis are achieved using contact thermal devices such as a blended cut/coagulation setting, bipolar circumactive probe cautery, and non-energy devices such as clips and band ligation (12).
A suction technique can also be used to isolate the tumor. It uses a multiband mucosectomy device and creates a pseudopolyp via band ligation. This cap-assisted EMR technique can be deployed to resect the tumor with or without submucosal fluid separation. It uses a cylindrical cap (circular or oblique) pre-loaded onto the endoscope to help suction and trap the mucosa into the cap followed by resection via a hot snare.
Indications for EMR are reserved for tumors in the submucosa that are often less than 2 cm in size and are enlarging or symptomatic. Larger lesions carry the risk of perforation and may need to be resected in a piecemeal fashion. It has the advantages of its noninvasive nature, decreased procedure time, and cost effectiveness. Major drawbacks of EMR include difficulty with en bloc resection of larger tumors, uncontrolled bleeding occurring at rates ranging from 0–24%, and perforation at a risk of 0.7–2.5% (13). A literature review of EMR use in BET resection is demonstrated in Table 1.
Table 1
| Study | Study period | Tumor location | N | Tumor size (mm) | Operation time | Additional techniques | En-bloc | Complications | Pathology | Recurrence | Follow-up (month) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hyun, Endoscopy, 1998 | Not specified | Esophagus | 62 | 19 [6–75] | NA | Snare polypectomy | 98.3% | 3 Delayed Bleeding | 56 leiomyomas; 4 GCT; 1 Neurogenic tumor; 1 cyst | 0% | 38.4 [3–107] |
| Battaglia, Surg Endoscopy, 2006 | 2000–2004 | Esophagus | 6 | NA | NA | Cap and Snare | 100% | None | 6 GCT | 0% | 36 |
| Kahng, Surg Endosc, 2013 | 2007–2011 | Esophagus | 22 | 8.65±4.1 | 14.9±6.8 | EMR with ligation EMR Snare | 92.6% | 3 Delayed Bleeding | 20 GCT | 0% | 15 [9–31] |
| Nie, Int J Clin Exp Pathol, 2014 | 2003–2013 | Esophagus | 12 | 2–28 | NA | NA | 100% | 1 Bleeding | 12 GCT | 0% | 18 [2–54] |
| Sharma, J Gastrointest Canc, 2017 | 2017 | Esophagus | 4 | 4.8±2.0 | NA | EMR- Duette method | NA | None | 4 GCT | 0% | 19.5±5.0 |
| Cakar, Instanbul Med J, 2020 | 2013–2017 | Esophagus | 4 | 9±4.2 | NA | NA | 100% | None | 4 Leiomyomas | 0% | 45±22.2 |
Data are presented as median (interquartile range) or mean ± SD. EMR, endoscopic mucosal resection; GCT, granulosa cell tumor.
Endoscopic submucosal dissection (ESD)
ESD was developed to address the limitations of EMR to adequately resect benign tumors of larger size (14). The technique utilizes an endoknife which ranges from basic Hook and Dual Knife, scissor-like knife, insulation Tip knife with better maneuverability to knives with a water-flush function to enhance submucosal dissection with fluid. After visualization of the submucosal tumor, markings are made to delineate the extent of the resection. The submucosal layer is separated from the MP via a submucosal injection. The mucosal incision is then performed 2–3 mmfrom the proximal or distal edge of the tumor, depending on the type of endoknife used, and the lesion is separated from the mucosa using a blended endocut/spray coagulation technique to achieve hemostasis (15). The submucosal dissection is then propagated to the lesion via a bidirectional mucosal tunnel created (16) or using a “clip-w/lines” method (17) to provide traction and counter-traction to separate the submucosal layers. Water jets have been purported as useful tools to detect potential breach of the MP and closure via easily manageable clips. They can be useful for securing the field of view during bleeding and can sometimes be utilized as an alternative method for dissecting during ESD.
An important complication of ESD and EMR is perforation and breach into the deeper esophageal layer which can subsequently cause mediastinal emphysema. If unresolved, the latter may lead to full-thickness perforation, pneumothorax, and potentially shock. These potential hemodynamic complications make careful vital signs monitoring and communication with anesthesia critical. Perforation rates for ESD have been reported to be as high as 6.4%, three times greater than EMR (18). Long-term complications of ESD include strictures. The likelihood of stricture increases if three-quarters, or more, of the circumference of the overlying mucosa, is incised. Techniques such as prophylactic endoscopic balloon dilation, oral prednisolone, intralesional triamcinolone, and ex-vivo epithelial sheet layers transplantation have been described to reduce perforation rates with positive results (19).
ESD has significantly improved the complete resection rate for esophageal and gastrointestinal neoplasms without the need for a piecemeal resection, indiscriminate of tumor size. It is important to note that the ESD is more technically complex with longer procedural time and the esophagus presents a unique challenge in the dissection process due to a narrow lumen and need for significant device manipulation, especially in tumors located nearest to the GE junction (20). A literature review of ESD applications in BET resection is demonstrated in Table 2.
Table 2
| Study | Study period | Tumor location | N | Tumor size (mm) | Operation time (min) | ESD Knives used | En-bloc | LOS (days) | Complications | Pathology | Recurrence | Follow-up (month) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shi, Gastrointest Endosc, 2011 | 2008–2010 | Esophagus | 28 | 10.3±7.0 | 73.5 [30–120] | Hybrid Knife | 93.3% | 3.9 (0–11) | 2 Perforations | 27 Leiomyomas 1 GIST | 0% | 12 [3–27] |
| Quan Lin, Gastrointest Endosc, 2012 | 2007–2011 | Esophagogastric-Junction | 143 | 17.6 [5–50] | 45.1 [7–120] | Electrosurgical knife, needle knife, Hook knife | 94.4% | 2.8 [1–8] | 6 perforations | 121 Leiomyoma; 20 GIST; 1 GCT 1 Intramuscular Lipoma | 0% | 21.1 [6–48] |
| Wang, Surg Endosc, 2013 | 2009–2011 | Esophagus | 21 | 30.0±4 | 87.2±7.7 | Hook knife | 5.7±1.0 days | 4 Bleeding 3 Perforation 1 Mediastinal Infection | 21 Leiomyomas | 0% | 26 | |
| Lu, World J Surg Onc, 2014 | 2006–2011 | Esophagus | 14 | 12.1 [4–26] | 38.2±10.1 | Hook Knife | 92.9% | NA | 0% | 14 GCT | 0% | 16.6±12.7 |
| Nie, Int J Clin Exp Pathol, 2014 | 2003–2013 | Esophagus | 17 | 2–28 | NA | Not specified | 100% | NA | 1 Bleeding | 17 GCT | 0% | 18 [2–54] |
| Cakar, Instanbul Med J, 2020 | 2013-2017 | Esophagus | 14 | 11.8±6.6 | NA | NA | NA | NA | 1 Delayed bleeding | 14 Leiomyomas | 0% | 45±22.2 |
Data are presented as median (interquartile range) or mean ± SD. ESD, endoscopic submucosal dissection; LOS, length of stay; GCT, granulosa cell tumor; GIST, gastrointestinal stromal tumor.
Submucosal tunneling endoscopic resection (STER)
The management of BET lodged deeper into the MP tends to be more invasive. Traditional open and laparoscopic surgeries are associated with higher invasiveness and morbidity and maybe even increased mortality. STER involves entry into submucosal space away from the tumor, submucosal tunneling, circumferential mobilization of the tumor followed by resection, retrieval, and closure of the mucosal opening at the end. In one technique, after identifying and marking the circumferential border of the esophageal tumor, a mixture of 10 mL saline and 0.2% indigo carmine is injected at least 6 cm proximal to the tumor (21). A 2 cm longitudinal incision of the mucosal layer is made away from the tumor. With a transparent cap attached to the tip of the endoscope, the submucosal layer is dissected from the MP. The tunnel is extended 2 cm past the distal tumor border and after full exposure of the tumor, the latter is then dissected free using a hybrid knife (Figure 2).The tumor is extracted out through the tunnel into the esophageal lumen and out of the patient’s mouth. The mucosotomy opening is closed using clips or endoscopic sutures (22).
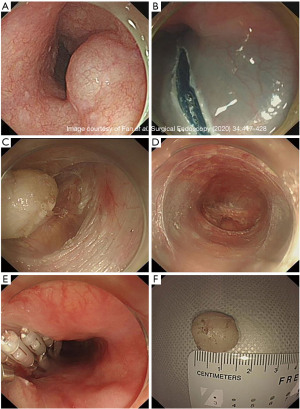
STER technique is an efficient and safe procedure for esophageal tumors involving the MP. The use of submucosal tunneling has the main advantage of minimizing mucosal injury and improved wound healing and infections post-operatively. A recent meta-analysis has shown complete resection rates of 97.5%, with no tumor recurrence. Given the invasive nature of STER, gas dissection causing pneumothorax, pneumomediastinum, subcutaneous emphysema, or pneumoperitoneum has been estimated at 14.8%, with the majority of them resolving with conservative therapy (23). A literature review of STER use in esophageal benign tumors resection is demonstrated in Table 3.
Table 3
| Study | Study period | Tumor location | N | Tumor size (mm) | Operation time (min) | Closure method | Technical success | En bloc | R0 | LOS (day) | Complications | Pathology | Recurrence | Follow-up (month) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inoue, Endoscopy, (2012) | N/A | Esophagus, Cardia | 9 | 12 [12–30] | 93 [84–365] |
Clips | 78% | 100% [2/9 aborted] | 100% | 4 [4–16] | None | 1 GIST, 5 Leiomyomas, 1 aberrant pancreas | N/A | N/A |
| Gong, Endoscopy, (2012) | Jun 2011–Nov 2011 | Esophagus, Cardia | 12 | 19 [10–40] | 48 [30–60] | Clips | 100% | 83% | N/A | N/A | 2 PTX | 7 GIST, 5 Leiomyomas | N/A | N/A |
| Xu, Gastrointestinal Endoscopy, (2012) | Jun 2010–Mar 2011 | Esophagus, Cardia, Stomach | 15 | 19 [12–25] | 79 [25–130] | Clips | 100% | 100% | 100% | 3.8 [3–5] | 1 PTX | 5 GIST, 9 Leiomyomas, 1 glomus tumor | None | 1-6 |
| Liu, Surgical Endoscopy, (2013) | Apr 2011–Jun 2012 | Esophagus, Cardia, Stomach | 12 | 19 [10–30] | 78 [50–130] | Clips | 100% | 100% | 100% | N/A | 4 PTX, 2 Pleural effusions | 2 GIST, 9 Leiomyomas, 1 Schwannoma | None | 5 [2-15] |
| Ge, Endoscopy Ultrasound, (2013) | Oct 2009–Dec 2011 | Esophagus | 17 | 24 [12–50] | 97 [60–150] | Clips | 100% | N/A | N/A | N/A | None | 1 GIST, 16 Leiomyomas | None | 7 [3-13] |
| Wang, Surgical Endoscopy, (2013) | Nov 2009–Nov 2011 | Esophagus | 18 | 33 [21–45] | 68 | Clips | N/A | N/A | N/A | N/A | 3 Bleeding | 18 Leiomyomas | None | 17 |
| Wang, Surgical Endoscopy, (2014) | Jul 2010–Aug 2012 | GEJ | 57 | 22 [6–35] | 47 [15–120] | Clips | 100% | 100% | NA | 2.7 [2–6] | 5 PTX, 2 Pleural effusions | 7 GIST, 46 Leiomyomas, 2 Schwannoma, 1 lipoma, 1 granular cell tumor | None | 12 [6-24] |
| Ye, Surgical Endoscopy, (2014) | Aug 2011–Feb 2013 | Esophagus, Cardia, Stomach | 85 | 19 [10–30] | 57 [30–115] | Clips | 100% | 100% | NA | 5.9 [2–14] | 6 PTX | 19 GIST, 65 Leiomyomas, 1 calcifying fibrous tumor | None | 8 [2-19] |
| Lu, Surgical Endoscopy, (2014) | Jan 2010–Jan 2014 | Esophagus, Cardia | 45 | 12 | 84 | Clips | 100% | 98% | 98% | NA | None | 3 GIST, 42 Leiomyomas | None | 9±9 |
| Zhou, World J Gastroenterology, (2015) | Aug 2012–Oct 2013 | GEJ | 21 | 23 [10–40] | 63 [45–90] | Clips | 100% | 86% | NA | 4.3 [3–7] | 1 Pleural effusion | 6 GIST, 15 Leiomyomas | None | 6 [2-14] |
| Tu, Gastroenterology Research and Practice, (2018) | Sep 2011–May 2018 | Esophagus | 115 | 19.4 [8–60] | 46.7 [10–150] | Clips | N/A | 98% | 100% | 5.9 [3–15] | 9 Perforations, 2 PTX, 9 SubQ emphysema | 5 GIST, 113 Leiomyomas, 1 Granular Cell tumor | None | 15 [1-71] |
| Chen, Surgical Endoscopy, (2020) | Jan 2011–Dec 2017 | Esophagus | 90 | 21±12.7 | 43 [31.8–63.0] | Clips | N/A | N/A | 90% | 5 [4–6] | SubQ emphysema, hemorrhage | 1 GIST, 87 Leiomyomas, 1 Granular Cell tumor, 1 Fibroma | 4.44% | 11.5 [1-77] |
Data are presented as median (interquartile range) or mean ± SD. LOS, length of stay; PTX, pneumothorax; SubQ, subcutaneous; GIST, gastrointestinal stromal tumor.
Endoscopic submucosal excavation (ESE)
The ESE technique is a modification of endoscopic dissection primarily used for submucosal tumors located at the esophagogastric junction, cardia, and stomach. This procedure was created in 2008 to reduce the mucosal defect formed via a longitudinal incision. The procedure begins with argon electrocoagulation marking around the lesion. A saline solution mixed with 2% indigo carmine and 1% epinephrine is injected at multiple points around the lesion to lift the mucosa and provide a cushion in the submucosa. A longitudinal incision is made along the proximal and distal margins with a hook knife to open the mucosa and expose the underlying tumor (Figure 3). The tumor is then separated from the submucosal tissue and muscle fibers with an insulated tip knife. The tumor can then be removed. Piecemeal resection with snare can be performed if removing the tumor in one specimen is not possible. Any intraoperative bleeding encountered can be treated with hot biopsy forceps or argon plasma coagulation (22,24). Due to the removal of overlying mucosa, the defect can be difficult to close with endoscopic clips. The artificial ulcer created from this procedure is mandatory to reduce the risks of perforation, infection, and delay bleeding. A few cases have been reported using fibrin sealant over the artificial ulcer to help prevent these complications (25).
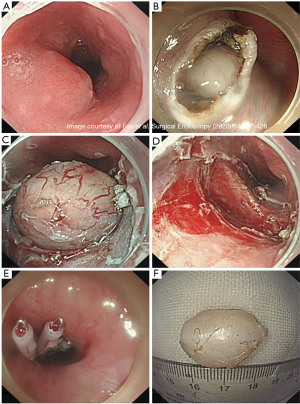
This procedure is beneficial for SMTs at the GEJ originating from the MP layer, as surgery in this area can cause deformities leading to gastroesophageal reflux or late stenosis (24). This procedure is easier to perform than STER. Increased operation times and complication rates are seen with larger tumor sizes (22). A literature review of ESE use in esophageal benign tumors resection is demonstrated in Table 4.
Table 4
| Study | Study period | Tumor location | N | Tumor size (mm) | Operation time (min) | Closure method | Technical success | En bloc | R0 | LOS (day) | Complications | Pathology | Recurrence | Follow-up (month) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zhang, Dig Dis Sci, (2013) | Dec 2008–Dec 2011 | GEJ | 68 | 16.2 [7–35] | N/A | Clips | N/A | N/A | 96% | 5 [2–9] | Perforation | 29 GIST, 39 Leiomyoma | N/A | 23 [6-42] |
| Lu, Surgical Endoscopy, (2014) | Jan 2010–Jan 2014 | Esophagus, Cardia | 35 | 11.5±3.7 | 65.9±18.0 | Clips | N/A | N/A | 95% | N/A | Perforation x4 | 6 GIST, 32 Leiomyoma | None | 28.3 ± 6.5 |
| Chen, Surgical Endoscopy, (2020) | Jan 2011–Dec 2017 | Esophagus | 77 | 16.8±15.4 | 30 [21.5–59.5] | Clips | N/A | N/A | 89.6% | N/A | SubQ emphysema | 4 GIST, 73 Leiomyoma | 2.60% | 18 [1-80] |
Data are presented as median (interquartile range) or mean ± SD. LOS, length of stay; N/A, not available; GIST, gastrointestinal stromal tumor.
Endoscopic full-thickness resection (EFTR)
EFTR requires a breach of the MP to fully resect the submucosal tumors. The “exposed” and “non-exposed” approaches are delineated by the sequence of resection and closure. The “exposed” or “standard” EFTR approach is initiated with resection first, exposure of luminal space from the mediastinal space, and closure of the defect (20). While the “non-exposed” approach reinforces the potential defect prior to resection. Esophageal lesions undergo the exposed EFTR approach when faced with reduced maneuverability of the lumen. In standard EFTR, the submucosa is initially dissected at the submucosal plane using fluid expansion (19). The SMT is marked and incised circumferentially which allows for en-bloc resection with electrosurgical knives. In cases where the SMT heavily involves the MP, the dissection is continued through the entirety of the MP i.e., full-thickness. Defect closure techniques include the loop-and-clip technique, which is used for >2 cm defects, while smaller defects can undergo endoscopic suturing devices or cap-mounted clips (26).
EFTR technique is an adaptation of the ESD for tumors encompassing the MP. In comparison to STER, EFTR has been noted to have longer suture time, a higher number of closure device usage such as clips, and longer hospital stay. A literature review of EFTR use in esophageal benign tumors resection is demonstrated in Table 5.
Table 5
| Study | Study period | Tumor location | N | Tumor size (mm) | Operation time | Closure method | Technical success | En bloc | R0 | LOS (day) | Complications | Pathology | Recurrence | Follow-up (month) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zhu, Journal of Cardiothoracic Surgery, (2019) | Jan 19 | Esophagus | 1 | 5 cm | N/A | Clips | N/A | 100% | 100% | N/A | None | Leiomyoma | N/A | 2 |
| Muramoto, Digestive Endoscopy, (2020) | Dec 19 | Esophagus | 1 | 3 cm | 80 min | Clips | N/A | 100% | 100% | 7 days | None | GCT | N/A | N/A |
LOS, length of stay; N/A, not available; GCT, granulosa cell tumor.
It is important to recognize that counter-traction is essential in the endoscopic treatment of esophageal tumors since in contrast to surgery, there is no access for an assistant ‘hand’ to provide traction making identification of the dissection planes difficult. On some occasions, the weight of the tumor can be utilized as a traction force during excision. Various traction methods and assistive devices have been reported in literature as attempts to improve lesion exposure and aid in dissection. A simple technique can entail the use of silk line connected to a clip attached to the lesion to provide exposure. However, this provides only pulling traction and requires space making it harder to utilize for esophageal lesions. Methods of traction that allow both pushing and pulling include the use of forceps anchored to the edge of the lesion through the accessory port. The Endolifter is a novel grasping forceps incorporated within a transparent plastic cylinder at the end of the scope that has been utilized for mucosa lifting and dissection in endoscopy (27). The double endoscope technique has been also described where a second smaller endoscope is inserted within the main scope with grasping forceps. Lastly, other potential methods are still under investigation including magnetic traction where intraluminal magnets attached to the lesion are manipulated using an external magnet.
Additional accessory and assistance devices are in continuous developments in endoscopy. This filed has been rapidly developing in the last years to improve efficacy and safety of endoscopic resection of gastrointestinal tumors. Harlow et al. have reported a recent narrative review on tools and accessories that have been introduced to ESD including endoscopic knives, needle types, injection solutions, and hemostatic devices (27).
There were limitations to this study that were inherent to its retrospective nature. Perioperative factors including patients’ comorbidities and operator experience were not reported by the majority of the studies. Included studies were mostly case series and retrospective studies with small patients’ numbers risking reporting bias and making generalizability of the study findings difficult. Follow-up was variable among the studies with limited long-term data. We, however, describe the aforementioned techniques and provide a comprehensive review of the available literature regarding endoscopic resection of BET suggesting an algorithm that can be utilized in clinical decision making.
Conclusions
Several endoscopic techniques have been described for the resection of BET with a good safety profile and variation in their utility and outcomes. Larger scale prospective studies are required for assessment of their efficacy, complications, and long-term outcomes.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Abbas E. Abbas and Roman V. Petrov) for the series “New Technologies in Esophageal Surgery and Endoscopy” published in Annals of Esophagus. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-32/rc
Peer Review File: Available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-32/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-32/coif). The series “New Technologies in Esophageal Surgery and Endoscopy” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Choong CK, Meyers BF. Benign esophageal tumors: introduction, incidence, classification, and clinical features. Semin Thorac Cardiovasc Surg 2003;15:3-8. [Crossref] [PubMed]
- Lewis RB, Mehrotra AK, Rodriguez P, et al. From the radiologic pathology archives: esophageal neoplasms: radiologic-pathologic correlation. Radiographics 2013;33:1083-108. [Crossref] [PubMed]
- PLACHTA A. Benign tumors of the esophagus. Review of literature and report of 99 cases. Am J Gastroenterol 1962;38:639-52. [PubMed]
- Seremetis MG, Lyons WS, deGuzman VC, et al. Leiomyomata of the esophagus. An analysis of 838 cases. Cancer 1976;38:2166-77. [Crossref] [PubMed]
- Ha C, Regan J, Cetindag IB, et al. Benign esophageal tumors. Surg Clin North Am 2015;95:491-514. [Crossref] [PubMed]
- Lee LS, Singhal S, Brinster CJ, et al. Current management of esophageal leiomyoma. J Am Coll Surg 2004;198:136-46. [Crossref] [PubMed]
- Zuccaro G Jr. The use of endoscopic ultrasound in esophageal disease. Gastroenterol Hepatol (N Y) 2007;3:163-4. [PubMed]
- Krill T, Baliss M, Roark R, et al. Accuracy of endoscopic ultrasound in esophageal cancer staging. J Thorac Dis 2019;11:S1602-9. [Crossref] [PubMed]
- Hatch GF 3rd, Wertheimer-Hatch L, Hatch KF, et al. Tumors of the esophagus. World J Surg 2000;24:401-11. [Crossref] [PubMed]
- Ko WJ, Song GW, Cho JY. Evaluation and Endoscopic Management of Esophageal Submucosal Tumor. Clin Endosc 2017;50:250-3. [Crossref] [PubMed]
- Kinney T, Waxman I. Treatment of benign esophageal tumors by endoscopic techniques. Semin Thorac Cardiovasc Surg 2003;15:27-34. [Crossref] [PubMed]
- Choi CW, Kang DH, Kim HW, et al. Endoscopic resection for small esophageal submucosa tumor: Band ligation versus conventional endoscopic mucosal resection. Medicine (Baltimore) 2017;96:e7574. [Crossref] [PubMed]
- ASGE Training Committee. Core curriculum for endoscopic mucosal resection. Gastrointest Endosc 2021;93:293-6. [Crossref] [PubMed]
- Ono S, Fujishiro M, Koike K. Endoscopic submucosal dissection for superficial esophageal neoplasms. World J Gastrointest Endosc 2012;4:162-6. [Crossref] [PubMed]
- Li B, Wang X, Zou WL, et al. Endoscopic resection of benign esophageal schwannoma: Three case reports and review of literature. World J Clin Cases 2020;8:5690-700. [Crossref] [PubMed]
- Technology status report evaluation. Endoscopic mucosal resection. Gastrointest Endosc 2000;52:860-3. [Crossref] [PubMed]
- Wang C, Wang Y, Li Y, et al. A Clip-with-Line Traction Suture Method for Closing Mucosal Defects after Endoscopic Submucosal Dissection. Gastroenterol Res Pract 2021;2021:8817726. [Crossref] [PubMed]
- Dellatore P, Bhagat V, Kahaleh M. Endoscopic full thickness resection versus submucosal tunneling endoscopic resection for removal of submucosal tumors: a review article. Transl Gastroenterol Hepatol 2019;4:45. [Crossref] [PubMed]
- Kang MS, Hong SJ, Han JP, et al. Endoscopic submucosal dissection of a leiomyoma originating from the muscularis propria of upper esophagus. Korean J Gastroenterol 2013;62:234-7. [Crossref] [PubMed]
- Zhu S, Lin J, Huang S. Successful en bloc endoscopic full-thickness resection of a giant cervical esophageal leiomyoma originating from muscularis propria. J Cardiothorac Surg 2019;14:16. [Crossref] [PubMed]
- Wadhwa V, Franco FX, Erim T. Submucosal Tunneling Endoscopic Resection. Surg Clin North Am 2020;100:1201-14. [Crossref] [PubMed]
- Chen Y, Wang M, Zhao L, et al. The retrospective comparison between submucosal tunneling endoscopic resection and endoscopic submucosal excavation for managing esophageal submucosal tumors originating from the muscularis propria layer. Surg Endosc 2020;34:417-28. [Crossref] [PubMed]
- Chen T, Zhou PH, Chu Y, et al. Long-term Outcomes of Submucosal Tunneling Endoscopic Resection for Upper Gastrointestinal Submucosal Tumors. Ann Surg 2017;265:363-9. [Crossref] [PubMed]
- Zhang Y, Ye LP, Zhu LH, et al. Endoscopic muscularis excavation for subepithelial tumors of the esophagogastric junction originating from the muscularis propria layer. Dig Dis Sci 2013;58:1335-40. [Crossref] [PubMed]
- Du C, Chai N, Linghu E, et al. Treatment of cardial submucosal tumors originating from the muscularis propria layer: submucosal tunneling endoscopic resection versus endoscopic submucosal excavation. Surg Endosc 2018;32:4543-51. [Crossref] [PubMed]
- Xiu H, Zhao CY, Liu FG, et al. Comparing about three types of endoscopic therapy methods for upper gastrointestinal submucosal tumors originating from the muscularis propria layer. Scand J Gastroenterol 2019;54:1481-6. [Crossref] [PubMed]
- Harlow C, Sivananthan A, Ayaru L, et al. Endoscopic submucosal dissection: an update on tools and accessories. Ther Adv Gastrointest Endosc 2020;13:2631774520957220. [Crossref] [PubMed]
Cite this article as: Alwatari Y, Ayalew D, Sevdalis AE, Scheese D, Vudatha V, Julliard W, Shah RD. Endoscopic resection techniques of benign esophageal tumors: literature review. Ann Esophagus 2023;6:17.