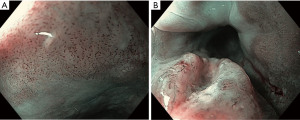
Indications for endoscopic treatment of adenocarcinoma and squamous cell cancer of the esophagus
Introduction
Esophageal cancer can be differentiated in two subtypes: esophageal adenocarcinoma (EAC) and esophageal squamous cell carcinoma (ESCC). Although a rising incidence of EAC could be detected in the Western world during the last decades, ESCC remains the most common carcinoma of the esophagus worldwide with a percentage of over 90% (1). Most studies on ESCC have been published from Asian countries (2).
Diagnosis and treatment of early stage esophageal carcinoma can be challenging.
This article gives an overview over current endoscopic treatment options of early stage EAC and ESCC.
EAC
Early stage EAC and lymph node metastasis
Compared to ESCC, the risk of lymph node metastasis in early stage EAC is lower. The risk of lymph node metastasis in EAC increases with the depth of submucosal invasion
A recent study from Japan showed no lymph node metastasis in 32 patients with EAC and a submucosal invasion depth <500 µm, a lesion size less than 30 mm and no high-risk factors such as lymphatic invasion or poor differentiation (3). Similar results were published in 2013 by Manner et al., 66 patients with suspected EAC received primary endoscopic resection (4). All patients fulfilled low risk criteria: macroscopically polypoid or flat with histologic findings of initial submucosal invasion (sm1), good-to-moderate tumor differentiation (G1–2) and no tumor invasion into lymphatic vessels or blood vessels. Lymph node metastasis were only reported in 1 patient (4).
Endoscopic detection
The detection of early stage lesions with white light imaging (WLI) can be challenging. Endoscopic screening should be performed by using the combination of WLI, image enhancement techniques and chromoendoscopy such as staining with acetic acid or combined with indigo carmine (5).
Image enhancement technologies
New technological advances, such as digital light filter (narrow band imaging = NBI, Olympus) or endoscopic post-processing technology (Fujinon intelligent Chromoendoscopy = FICE, Fujinon; iSCAN, Pentax) offer a “virtual” chromoendoscopy during examination. These diagnostic features highlight superficial vasculature and the mucosal pattern and their changes during carcinogenesis und consecutive neovascularization.
The combination of new enhancement techniques and standard WLI can be helpful to achieve a complete endoscopic resection by enabling better recognition of the lateral margins of the lesion (5).
Chromoendoscopy with acetic acid
Four-quadrant biopsies every 2 cm and additional biopsies of all visible abnormalities in Barrett’s esophagus is recommended in Western guidelines (6-8).
Chromoendoscopy with acetic acid stains non-dysplastic Barrett’s mucosa white and enhances surface patterns, making it easier to predict dysplastic areas (6,7). A recent feasibility trial published by the ABBA study group compared neoplasia detection rates for nontargeted biopsies (Seattle protocol) versus acetic acid-targeted biopsies (Porthsmouth protocol) (8). Neoplasia prevalence was 4.7% (9/192) and the number of biopsies needed to diagnose neoplasia was much higher using the Seattle protocol than when using the Portsmouth protocol (8). A fully empowered study is yet come.
BING classification for Barrett’s esophagus
Characterization of mucosal and vascular pattern is an endoscopic tool for the differentiation of regular Barrett’s mucosa from dysplastic areas in Barrett’s esophagus by using NBI (9,10).
In 2015, Sharma et al. introduced the BING classification for Barrett’s esophagus. The pattern is differentiated in mucosal and vascular, furthermore in regular or irregular (Table 1). This classification showed high diagnostic accuracy with a sensitivity of 80.4% and a specificity of 88.4% (10,11).
Table 1
| Morphologic characteristics | Classification |
|---|---|
| Mucosal pattern | |
| Circular, ridged/villous, or tubular patterns | Regular |
| Absent or irregular patterns | Irregular |
| Vascular pattern | |
| Blood vessels situated regularly along or between mucosal ridges and/or those showing normal, long, branching patterns | Regular |
| Focally or diffusely distributed vessels not following normal architecture of the mucosa | Irregular |
NBI, narrow band imaging.
Artificial intelligence (AI) for detection of Barett’s cancer
Recently, Ebigbo et al. showed that AI has the potential to differentiate non-neoplastic Barrett’s mucosa from EAC with a sensitivity of 97% and a specificity of 88% for WLI and a sensitivity of 94% and specificity of 80% for NBI images (12).
Meanwhile the same group could demonstrate for the first time, that real-time detection of Barrett’s cancer is possible with AI (13).
Endoscopic treatment
Endoscopic treatment of early stage esophageal neoplasia has gained increasing acceptance over the last decades. Compared to invasive surgical procedures such as esophagectomy with lymph node dissection, endoscopic resection techniques are associated with a lower mortality and morbidity rate (14).
Endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) are well accepted endoscopic resection techniques.
According to the European Society of Gastroenterology (ESGE) guidelines, endoscopic en-bloc resection should be the treatment of choice for high-grade dysplasia or mucosal EAC without lymphatic or vascular invasion and differentiation grades 1 to 2 (5). These criteria might be extended if submucosal invasion is less than ≤500 µm and the resected carcinoma is well or moderately differentiated, with a lesion size <3 cm and without lymphovascular invasion (4,5).
If these conditions are not fulfilled, additional surgical treatment is recommended.
ESD
Today, ESD is a well-established treatment option for early stage esophageal neoplasia. It allows an oncologically accurate histopathological assessment in terms of R0-situation and shows lower recurrence rates compared to EMR (Figure 1).
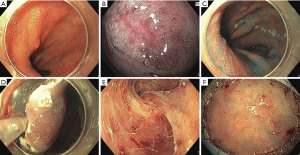
Though the technique is time consuming and the learning curve is flat, procedure time has decreased over the last years and complications (e.g., bleeding, perforation and stricture formation) can be managed endoscopically (15,16).
The ESGE guideline recommends ESD for lesions >15 mm, lesions suspicious for submucosal invasion or lesions with poor lifting (5).
A meta-analysis out of 11 studies on ESD in Barret’s esophagus showed a R0-resection rate of 92.9% (17). European data could reach R0 resection rates in some studies in more than 90% of the cases with a recurrence rate of 2.4% after 3 months (18).
EMR
EMR is recommended for lesions <15 mm if en bloc resection can be assured (5).
ESD vs. EMR
There are only few studies comparing ESD and EMR for the treatment of Barrett’s esophagus. Terheggen et al. published a prospective multicenter trial 2017. ESD achieved higher R0 resection rates (10/17 vs. 2/17 in the EMR group), there was no significant difference in remission at 3 months. However, there are limitations in this study such as the small sample size as well as the small size of lesions included. Interestingly the perforation rate with ESD was unacceptable high with 10% and the R0-resection rate of ESD lower compared to other ESD studies.
Local ablative procedures
After endoscopic resection, remaining Barrett segments should be treated with local ablative procedures such as radiofrequency ablation (RFA), (hybrid-)argon plasma coagulation (APC) or other ablative techniques (e.g., cryotherapy) (5).
Published data showed metachronous lesions of up to 30% in 3 years, if the remaining Barrett segment was not removed (19,20).
In patients with high-grade dysplasia in Barrett’s esophagus with no visible lesion ablation is recommended. If low-grade dysplasia is histologically proven and a lesion is visible, either ablation or surveillance is recommended (21,22).
Squamous cell cancer
As mentioned above, ESCC remains the most common esophageal cancer worldwide with risk factors e.g., smoking, consumption of alcohol or radiation-induced carcinoma (23,24). In more than 50 percent, ESCC is diagnosed in advanced and endoscopically unresectable stages (18).
Early stage squamous cell cancer and lymph node metastasis
Evaluation of invasion depth is necessary according to the Japanese Society for Esophageal Diseases. The differentiation is made between mucosal (m1-m3) and submucosal invasion depth (sm1-sm3). An increasing risk of lymph node metastasis (LNM) depending on the invasion depth could be shown, from no LMN in m1-lesions up to 45.9% LNM in sm3-lesions (25). Other independent risk factors for lymph node metastasis are angioinvasion and tumor grading. These points are important to decide, if endoscopic resection is sufficient or if surgery is necessary.
Endoscopic detection
As in EAC, endoscopy of the esophagus should be performed by using the combination of WLI (conventional und high-definition), image enhancement techniques and chromoendoscopy (using Lugol’s iodine for ESCC).
Image enhancement techniques
Image enhancement techniques such as NBI, i-Scan and FICE are used during the examination of ESCC. NBI should be used to detect neoplasia additional to conventional chromoendoscopy with Lugol’s iodine (26).
Chromoendoscopy with Lugol’s iodine
Spraying the esophagus with Lugol’s iodine is a diagnostic tool for the detection and delineation of ESCC. Compared to normal squamous epithelium, there is a loss of glycogen in neoplastic areas. As Lugol’s iodine adheres to glycogen, the neoplastic area remains unstained; this is known as the “pink color sign” (27). The detection of ESCC can be improved significantly by using Lugol’s iodine. Because of the fact that inflammatory areas also remain unstained, sensitivity and specificity is low. Some patients reported nausea and chest pain after the use of Lugol’s iodine (28).
The JES-classification: prediction of the invasion depth of ESCC
The JES classification is a simplified classification for the magnified endoscopic evaluation of ESCC which was published by Oyama et al. in 2018 (29). This classification is based on the Inoue and Arima classifications. By judging the microvascular morphology, observed by NBI and magnifying endoscopy, a prediction of invasion depth becomes possible (Table 2).
Table 2
| Type of vessels | Definitions | Prediction of invasion depth | Histological assessment |
|---|---|---|---|
| A | Normal intracapillary loops or abnormal microvessels without severe irregularity | No invasion | Normal epithelium, inflammation, and LGIN |
| B | Abnormal microvessels with severe irregularity or highly dilated abnormal vessels | HGIN and invasive SCC | |
| B1 | Type B vessels with a loop like formation | T1a-epithelium or T1a-lamina propria mucosae | |
| B2 | Type B vessels without a loop-like formation | T1a-muscularis mucosae or T1b-submucosa | |
| B3 | Highly dilated vessels which calibers appear to be more than three times that of usual B2 vessels | T1b-SM2 or deeper |
JES, Japanese Esophageal Society; LGIN, low-grade intra-epithelial neoplasia; HGIN, high-grade intra-epithelial neoplasiaSCC, squamous cell carcinoma.
An accurate prediction of tumor invasion depth was possible in 90.5% of cases with type B vessels in 211 patients (Figure 2).
With regards to endoscopic resection, type B1 vessels are an absolute indication, type B2 vessels are a relative indication and type B3 vessels are a contraindication for endoscopic resection (29,30).
Endoscopic treatment options for early ESCC
EMR is an established treatment option for the resection of ESCC. Resection can be performed as a multiband EMR or cap assisted. Multiband-EMR is faster and cost-efficient, but both treatment options are efficacious (31). Limitations of EMR are reached if lesions exceed 15 mm in diameter, then it is impossible to achieve an en bloc resection and R0 situation. If the lesion is smaller than 15 mm, en bloc resection rates of about 53% have been reported (32). Therefore, the ESGE guideline recommends EMR in lesions smaller than 10 mm if en bloc resection is possible (5).
ESD vs. EMR
The advantages of ESD were mentioned above. Choosing the best endoscopic resection method is essential for the patient’s outcome. Though EMR is a safe, fast and cost-efficient treatment option for ESCC, ESD is associated with a higher R0 resection rate and lower recurrence rates compared to EMR (32). Takahashi et al. published a retrospective single center study with 300 patients suffering from ESCC. A total of 184 patients were treated by EMR, 116 patients underwent ESD. The R0 resection rate was 100% in the ESD group compared to 53% in the EMR group. Local recurrence was lower in the ESD group with 0.9% compared to 9.8% in the EMR group (2).
Local ablative procedures
Ablative procedures such as APC and RFA as well as photodynamic therapy have been discussed in the literature. Data are disappointing in terms of complete remission (33,34). The role of photodynamic therapy remains a salvage therapy if other therapy options are contraindicated (35).
Conclusions
Endoscopic resection techniques of early stage esophageal neoplasia have gained impact over the last years and detection of early esophageal neoplasia has increased. If detected early, curative endoscopic treatment is possible.
In EAC, endoscopic en bloc resection should be the treatment of choice for high-grade dysplasia, mucosal carcinoma and selected cancers with shallow submucosal invasion. The remaining Barrett’s mucosa should be treated with endoscopic ablation techniques
With regards to ESCC, small lesions can be treated effectively via EMR with low recurrence rates and high R0 rates. If a R0 situation can’t be reached with certainty by EMR, ESD should be the treatment option of choice. Low grade and high-grade dysplasia as well as ESCC limited to m1-m2 can be treated curatively with negligible to no risk of LMN.
Both tumor entities should be treated in centers with a high level of experience and expertise.
Finally, treatment strategies after non-curative resections should be discussed in a multidisciplinary setting, depending on the histopathological criteria and the patient’s status.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Hon Chi Yip and Philip Wai-Yan Chiu) for the series “Endoscopic Diagnosis and Treatment of Early Esophageal Cancer” published in Annals of Esophagus. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoe.amegroups.com/article/view/10.21037/aoe-2020-35/coif). The series “Endoscopic Diagnosis and Treatment of Early Esophageal Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Brown LM, Devesa SS, Chow WH, et al. Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. J Natl Cancer Inst 2008;100:1184-7. [Crossref] [PubMed]
- Takahashi H, Arimura Y, Masao H, et al. Endoscopic submucosal dissection is superior to conventional endoscopic resection as a curative treatment for early squamous cell carcinoma of the esophagus (with video). Gastrointest Endosc 2010;72:255-64, 264.e1-2.
- Ishihara R, Oyama T, Abe S, et al. Risk of metastasis in adenocarcinoma of the esophagus: a multicenter retrospective study in a Japanese population. J Gastroenterol 2017;52:800-8. [Crossref] [PubMed]
- Manner H, Pech O, Heldmann Y, et al. Efficacy, safety, and long-term results of endoscopic treatment for early stage adenocarcinoma of the esophagus with low-risk sm1 invasion. Clin Gastroenterol Hepatol 2013;11:630-5; quiz e45. [Crossref] [PubMed]
- Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2015;47:829-54. [Crossref] [PubMed]
- Lambert R, Rey JF, Sankaranarayanan R, et al. Magnification and chromoscopy with the acetic acid test. Endoscopy 2003;35:437-45. [Crossref] [PubMed]
- Guelrud M, Herrera I. Acetic acid improves identification of remnant islands of Barrett's epithelium after endoscopic therapy. Gastrointest Endosc 1998;47:512-5. [Crossref] [PubMed]
- Longcroft-Wheaton G, Fogg C, Chedgy F, et al. A feasibility trial of Acetic acid-targeted Biopsies versus nontargeted quadrantic biopsies during BArrett's surveillance: the ABBA trial. Endoscopy 2020;52:29-36. [Crossref] [PubMed]
- Alvarez Herrero L, Curvers WL, Bansal A, et al. Zooming in on Barrett oesophagus using narrow-band imaging: an international observer agreement study. Eur J Gastroenterol Hepatol 2009;21:1068-75. [Crossref] [PubMed]
- Sharma P, Bergman JJ, Goda K, et al. Development and Validation of a Classification System to Identify High-Grade Dysplasia and Esophageal Adenocarcinoma in Barrett's Esophagus Using Narrow-Band Imaging. Gastroenterology 2016;150:591-8. [Crossref] [PubMed]
- Ishihara R, Goda K, Oyama T, et al. Endoscopic diagnosis and treatment of esophageal adenocarcinoma: introduction of Japan Esophageal Society classification of Barrett's esophagus. J Gastroenterol 2019;54:1-9. [Crossref] [PubMed]
- Ebigbo A, Mendel R, Probst A, et al. Computer-aided diagnosis using deep learning in the evaluation of early oesophageal adenocarcinoma. Gut 2019;68:1143-5. [Crossref] [PubMed]
- Ebigbo A, Mendel R, Probst A, et al. Real-time use of artificial intelligence in the evaluation of cancer in Barrett's oesophagus. Gut 2020;69:615-6. [Crossref] [PubMed]
- Stein HJ, Feith M, Bruecher BL, et al. Early esophageal cancer: pattern of lymphatic spread and prognostic factors for long-term survival after surgical resection. Ann Surg 2005;242:566-73; discussion 573-5. [Crossref] [PubMed]
- Probst A, Golger D, Anthuber M, et al. Endoscopic submucosal dissection in large sessile lesions of the rectosigmoid: learning curve in a European center. Endoscopy 2012;44:660-7. [Crossref] [PubMed]
- Ebigbo A, Messmann H. How can we make the learning curve of endoscopic submucosal dissection for (Western) endoscopists less steep? Endoscopy 2016;48:697-8. [Crossref] [PubMed]
- Yang D, Zou F, Xiong S, et al. Endoscopic submucosal dissection for early Barrett's neoplasia: a meta-analysis. Gastrointest Endosc 2018;87:1383-93. [Crossref] [PubMed]
- Probst A, Aust D, Märkl B, et al. Early esophageal cancer in Europe: endoscopic treatment by endoscopic submucosal dissection. Endoscopy 2015;47:113-21. [Crossref] [PubMed]
- Peters FP, Kara MA, Rosmolen WD, et al. Endoscopic treatment of high-grade dysplasia and early stage cancer in Barrett's esophagus. Gastrointest Endosc 2005;61:506-14. [Crossref] [PubMed]
- Fleischer DE, Overholt BF, Sharma VK, et al. Endoscopic radiofrequency ablation for Barrett's esophagus: 5-year outcomes from a prospective multicenter trial. Endoscopy 2010;42:781-9. [Crossref] [PubMed]
- Weusten B, Bisschops R, Coron E, et al. Endoscopic management of Barrett's esophagus: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy 2017;49:191-8. [Crossref] [PubMed]
- Koop H, Fuchs KH, Labenz J, et al. S2k guideline: gastroesophageal reflux disease guided by the German Society of Gastroenterology: AWMF register no. 021-013. Z Gastroenterol 2014;52:1299-346. [Crossref] [PubMed]
- Zablotska LB, Chak A, Das A, et al. Increased risk of squamous cell esophageal cancer after adjuvant radiation therapy for primary breast cancer. Am J Epidemiol 2005;161:330-7. [Crossref] [PubMed]
- Lee CH, Wu DC, Lee JM, et al. Anatomical subsite discrepancy in relation to the impact of the consumption of alcohol, tobacco and betel quid on esophageal cancer. Int J Cancer 2007;120:1755-62. [Crossref] [PubMed]
- Bollschweiler E, Baldus SE, Schröder W, et al. High rate of lymph-node metastasis in submucosal esophageal squamous-cell carcinomas and adenocarcinomas. Endoscopy 2006;38:149-56. [Crossref] [PubMed]
- Lee CT, Chang CY, Lee YC, et al. Narrow-band imaging with magnifying endoscopy for the screening of esophageal cancer in patients with primary head and neck cancers. Endoscopy 2010;42:613-9. [Crossref] [PubMed]
- Inoue H, Rey JF, Lightdale C, et al. Lugol chromoendoscopy for esophageal squamous cell cancer. Endoscopy 2001;33:75-9.
- Kondo H, Fukuda H, Ono H, et al. Sodium thiosulfate solution spray for relief of irritation caused by Lugol's stain in chromoendoscopy. Gastrointest Endosc 2001;53:199-202. [Crossref] [PubMed]
- Oyama T, Inoue H, Arima M, et al. Prediction of the invasion depth of superficial squamous cell carcinoma based on microvessel morphology: magnifying endoscopic classification of the Japan Esophageal Society. Esophagus 2017;14:105-12. [Crossref] [PubMed]
- Japan Esophageal Society. Japanese Classification of Esophageal Cancer, 11th Edition: part II and III. Esophagus 2017;14:37-65.
- Zhang YM, Boerwinkel DF, Qin X, et al. A randomized trial comparing multiband mucosectomy and cap-assisted endoscopic resection for endoscopic piecemeal resection of early squamous neoplasia of the esophagus. Endoscopy 2016;48:330-8. [Crossref] [PubMed]
- Ishihara R, Iishi H, Takeuchi Y, et al. Local recurrence of large squamous-cell carcinoma of the esophagus after endoscopic resection. Gastrointest Endosc 2008;67:799-804. [Crossref] [PubMed]
- Tahara K, Tanabe S, Ishido K, et al. Argon plasma coagulation for superficial esophageal squamous-cell carcinoma in high-risk patients. World J Gastroenterol 2012;18:5412-7. [Crossref] [PubMed]
- He S, Bergman J, Zhang Y, et al. Endoscopic radiofrequency ablation for early esophageal squamous cell neoplasia: report of safety and effectiveness from a large prospective trial. Endoscopy 2015;47:398-408. [Crossref] [PubMed]
- McCann P, Stafinski T, Wong C, et al. The safety and effectiveness of endoscopic and non-endoscopic approaches to the management of early esophageal cancer: a systematic review. Cancer Treat Rev 2011;37:11-62. [Crossref] [PubMed]
Cite this article as: Fleischmann C, Probst A, Messmann H. Indications for endoscopic treatment of adenocarcinoma and squamous cell cancer of the esophagus. Ann Esophagus 2023;6:5.