
Endoscopic submucosal dissection for large early squamous cell carcinoma—traction assisted methods
Introduction
Surgical resection for esophageal cancer requires esophageal subtotal resection with lymph node dissection, as well as organ reconstruction using the stomach, large intestine or small intestine, which is extremely invasive to the body. Meanwhile, endoscopic resection is a minimally invasive treatment that only removes the mucosa of the esophagus and the incidence of procedural adverse events is lower than that for surgical resection (1,2). Endoscopic mucosal resection (EMR) was first performed for esophageal cancer in the late 1980s, and EMR with a cap-fitted panendoscope (EMR-C), a modified EMR method enabling treatment of larger lesions, was reported by Inoue et al. (3,4) in the early 1990s. However, because these methods used a snare, there were cases where the lesion was resected into pieces depending its location and size, resulting in incomplete endoscopic resection or additional surgical resection (5). In the late 1990s, endoscopic submucosal dissection (ESD) for gastric cancer appeared and has gradually gained popularity because of its accurate histological evaluation and favorable procedural outcomes (6-10). From Japan, it has been reported that the en bloc resection rate of ESD ranges from 95% to 100% and the complete resection rate from 88% to 94.6% (11-13). In addition, Yamashina et al. (14) reported long-term outcomes for 394 cases of superficial esophageal cancer treated by endoscopic resection, and the 5-year survival rate was 90.5% for epithelium/lamina propria mucosa, 71.1% for muscularis mucosae and 70.8% for submucosal cancer. Tsujii et al. (15) also reported that the 3- and 5-year recurrence-free survival rates after ESD were 91.5% and 84.8% in the curative resection group and 76.0% and 72.7% in the non-curative resection group, respectively. Furthermore, esophageal ESD is often performed as a total biopsy because of its diagnostic advantages and minimally invasive nature, and it is thought that diagnostic ESD will be further implemented in the future, based on the results of JCOG0508 (16). However, since the esophagus is located in the mediastinum and surgical intervention is invasive, adverse events related to esophageal ESD tend to be more severe than those in gastric ESD. Furthermore, esophageal ESD is thought to be more difficult and requires longer procedure times than gastric ESD because the esophagus has a narrow lumen and a thin muscular layer. To increase the use of esophageal ESD, it is essential to reduce the difficulty of the procedure. The traction-assisted approach overcomes some of the technical difficulties associated with esophageal ESD. Here, we review studies on these technical aspects.
Clip-with-thread method
During surgery, surgeons use their right and left hands for open abdominal surgery, and their skills have expanded to include laparoscopic surgery through multiple or single ports, and robotic surgery. In all of these surgical methods, there is a significant benefit to employing counter-traction with the other hand, clearly exposing the area of the surgical field and cutting plane for swift operation. In contrast, endoscopists are required to manage a perplexing situation single-handedly during ESD because there is no so-called “surgeon’s left hand” (17). Adequate visualization during ESD is essential for technical success and to reduce the incidence of adverse events. Applying vertical traction to the partially resected lesion significantly helps to maintain stable visualization. Oyama et al. (18) demonstrated a counter-traction method, the clip-with-thread (CT) method, for esophageal endoscopic treatment in which they attached a thread tied to a hemoclip to the lesion and achieved adequate visualization by pulling on the thread. Various other traction methods have been attempted to alleviate the difficulties of esophageal ESD. However, these interesting methods have not been widely adopted, because they are complicated, costly, or invasive (19-25). The modified CT method reported by Suzuki et al. (26) and Yoshida et al. (27,28) was simply to tie a length of commercially available dental floss (REACH○RR, Johnson & Johnson K.K., Tokyo, Japan) to the stainless-steel arm of the clip with a surgeon’s knot (Figure 1). The CT method is recognized as the simplest traction technique and has become the most popular method in Japan (29,30).
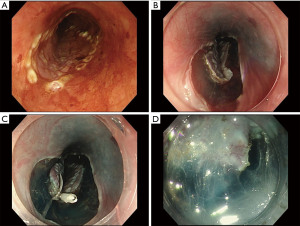
Two randomized controlled trials (RCT) and two retrospective comparative studies have been reported (summarized in Table 1). Koike et al. (32) conducted a randomised controlled trial (RCT) in 2015 comparing conventional ESD and the CT method in which the mean dissection time of the conventional ESD was 31.8 min, whereas that of the CT method was 19.8 min (P=0.044). Although the CT method seemed a promising treatment method with great potential to shorten the procedure time, the RCT had a small sample size and was limited to two operators at a single facility, requiring more solid evidence. In 2020, Yoshida et al. (34) reported a nationwide large-scale multicenter RCT in Japan (CONNECT-E study). CONNECT-E was a well-structured RCT and included lesions (117 in conventional ESD vs. 116 in the CT method) with a size of 20 mm or more, because small lesions (such as those 15 mm or smaller) are well managed by EMR-C (35,36). In the CONNECT-E study, the median ESD procedure duration was significantly shorter using the CT method compared with conventional ESD (44.5 vs. 60.5 min, P<0.001). Moreover, in six (5.2%) patients undergoing conventional ESD, the procedure was converted to the CT method to overcome technical difficulties arising during the procedure (perforation, n=3; prolonged procedure duration, n=3) and handover to another operator during ESD tended to occur more frequently in conventional ESD (6.0% vs. 0.9%, P=0.066). Although traction-related damage to the specimen was observed in 1.7% of the CT method, there was no significant difference between the two methods regarding the rate of horizontal margin involvement (10.3% vs. 6.9%, P=0.484). Additionally, the study analyzed the risk factors for experiencing technical difficulties, defined as follows: procedure duration >120 minutes; perforation; piecemeal resection; inadvertent incision; or handover to another operator. The results confirmed that the CT method reduced the risk of technical difficulties during ESD for large esophageal cancers (odds ratio: 0.265, 95% confidence interval: 0.094–0.649, P=0.005). Although there were no significant differences in the frequency of adverse events in the two RCTs, perforation was not observed with the CT method, indicating that the CT method is safer compared with conventional ESD. Ota et al. (31) and Xie et al. (33) also reported that the CT method had a lower rate of muscularis propria injury in their retrospective studies.
Table 1
| Authors | Year | Country | Design | Setting | Cases, n | Lesion size, mm | Circumferential extent, n | Procedure time, min | Volume of injection, ml | En bloc resection, % | R0 resection, % | Perforation, % | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <1/2 | ≥1/2 | ||||||||||||
| Ota et al. (31) | 2012 | Japan | Retrospective | Single center | 20/67 | 26.4/28.1* | 16/52 | 5/15 | 156/104* | – | – | – | 0/0 |
| (P=0.003) | (P=1.00) | ||||||||||||
| Koike et al. (32) | 2015 | Japan | RCT | Single center | 20/20 | 27.0/24.0* | 13/12 | 7/8 | 31.8/19.8*‡ | 7.5/2.6* | 100/100 | – | 0/0 |
| (P=0.044) | (P<0.001) | (P=1.000) | (P=1.00) | ||||||||||
| Xie et al. (33) | 2017 | China | Retrospective | Single center | 50/50 | 43.0/40.0 | 26/20 | 24/30 | 34.8/37.6*‡ | – | – | – | 0/0 |
| (P=0.252) | (P=1.00) | ||||||||||||
| Yoshida et al. (34) | 2020 | Japan | RCT | Multicenter | 117/116 | 30/30† | 75/75 | 42/41 | 60.5/44.5† | 40/30† | 99.1/100 | 87.2/91.4 | 4.3/0 |
| (P<0.001) | (P=0.001) | (P>0.99) | (P=0.30) | (P=0.60) | |||||||||
The results are shown in the order of publication of the control/study arm. RCT, randomized controlled trial; *Mean; †Median; ‡Dissection time. ESD, endoscopic submucosal dissection.
Submucosal tunneling method
When the size of the lesion is large, resected mucosa distally retracts the esophageal lumen during conventional ESD, which may cause an endoscopist to become disoriented. Moreover, rapid diffusion of the submucosal liquid cushion due to poor connective tissue of the submucosa of the esophagus worsens visualization of the submucosa, resulting in prolonged procedure times. To solve these technical difficulties, von Delius et al. (37) reported the submucosal tunneling (ST) method for the treatment of circumferential esophageal lesions in a live porcine model in 2007. The ST method pushes up the resected mucosa with an endoscope and facilitates the procedure by providing stable submucosal visualization and appropriate traction to the submucosa. Additionally, the ST method enables a stable scope position to be achieved inside the submucosal tunnel. The ST method is summarized in Figure 2. After submucosal injection, mucosal incisions were made at both the proximal and distal sides of the lesion, and the submucosa under the lesion was dissected to create a communication between the proximal and distal incisions.
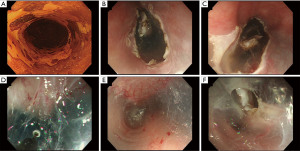
Two retrospective studies comparing conventional ESD and the ST method have been reported (Table 2) (40-42). In a propensity matching analysis by Huang et al. (38), there was a significant difference in the median procedural duration between conventional ESD and the ST method (48.0 vs. 38.0 min, respectively, P=0.006). Although there was no difference in the frequency of adverse events, a lower rate of muscular injury (52.6% vs. 28.9%; P=0.036) and a less frequent use of coagulation forceps (65.8% vs. 36.8%; P=0.012) was found with the ST method. The authors speculated that less frequent use of forceps might indicate that fewer major bleeding events occurred in the ST method. Another report by Zhang et al. (39) showed that the mean dissection speed was faster in the ST method than in conventional ESD (conventional: 16.10 vs. ST: 21.54 mm2/min, P=0.002). In both retrospective studies, en bloc resection rates and complete resection rates were similar in the two methods.
Table 2
| Authors | Year | Country | Design | Setting | Cases, n | Specimen size, mm | Circumferential extent, n | Procedure time, min | Dissection speed, mm2/min | En bloc resection, % | R0 resection, % | Perforation, % | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <3/4 | ≥3/4 | ||||||||||||
| Huang et al. (38) | 2017 | China | Retrospective | Single center | 38/38 | 36.0/39.0* | – | – | 48.0/38.9* | 17/23* | 100/100 | 94.7/100 | 7.9/0 |
| (P=0.006) | (P<0.001) | (P=1.000) | (P=0.152) | (P=0.077) | |||||||||
| Zhang et al. (39) | 2018 | China | Retrospective | Single center | 98/52 | 13.0/15.4†‡ | 72/29 | 26/23 | 92.4/93.2† | 16.1/21.5† | 88.8/96.1 | 86.7/84.6 | 1.0/1.9 |
| (P=0.944) | (P=0.002) | (P=0.126) | (P=0.722) | (P=0.646) | |||||||||
The results are shown in the order of publication of the control/study arm. *Median; †Mean; ‡Specimen area (cm2).
The ST method seems effective for large-size lesions, especially those with circumferential extent of 3/4 or more. However, bleeding inside the submucosal tunnel hampers visualization of the cutting plane, leading to longer procedure times and more adverse intraoperative events. Operators should carefully monitor submucosal vessels and avoid bleeding inside the submucosal tunnel.
Clip-with-thread method vs. submucosal tunneling method
Although there are no trials that directly compare the CT method with the ST method, Jin et al. (43) assigned 15 beginners to the CT method, the ST method, or the conventional method, respectively, in an animal study. The procedural time was the shortest using the CT method (47.4 min with the CT method, 67.0 min with the ST method, 67.0 min using the conventional method). The CT method had the lowest rate of esophageal perforation (CT: 5.0%, ST: 20.0%, Conventional: 40.0%). Furthermore, learning curves analysis showed that the CT method was the easiest for the trainees to master. Although further studies are required, it would seem unlikely that the ST method is superior to the CT method. Furthermore, the use of the ST method seems difficult for lesions with a circumferential extent of 1/2 or less.
Combination of the clip-with-thread and tunneling methods
The ST method improves the efficiency of the procedure for large lesions. However, the remaining submucosal layer is collapsed after the tunnel is created. Jacques et al. (44) conducted a prospective, single-arm study about the “tunnel + clip” strategy, a combination of ST with CT, and reported efficacy and safety even when performed by less experienced endoscopists. We also actively use a combination method for semi-circumferential or circumferential lesions in clinical practice as reported by Fraile-López et al. (45). The technical steps of the ST + CT method are as follows: (I) create a submucosal tunnel under the lesion as mentioned above; (II) withdraw the endoscope from the tunnel; (III) perform trimming of the submucosal layer from the proximal side; (IV) place the CT on the proximal side of the mucosa where the submucosa remains longitudinally; and (V) proceed with submucosal dissection. For full circumferential lesions, we create an additional tunnel on the opposite side to the first tunnel. This promising technique could allow proper tension to be applied to the collapsed submucosal layer. Further studies are strongly desired.
Future perspectives for esophageal ESD
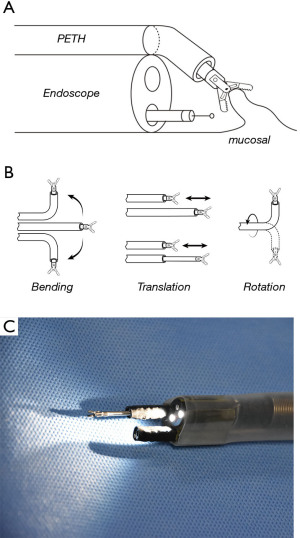
Recent advances in endoscopic equipment have enabled high complete resection rates of the esophagus. The traction assisted technique obviously facilitates esophageal ESD. However, it still has technical limitations in controlling the direction of the traction, adjusting the tension of the submucosal layer, and regrasping the tissue. To overcome these limitations, a robotic manipulation device is being developed. One or two robotic arms equipped with an endoscope enable holding of the tissue and provision of traction under the control of the operator (Figure 3). Hwang et al. (46) performed robotic arm-assisted ESD in the porcine model and reported faster dissection speeds compared with conventional ESD (122.3±76.5 vs. 47.5±26.9 mm2/min, P<0.001). Such a new endoscope equipped with “other hands” has substantial potential to alleviate technical difficulties in esophageal ESD. There is no doubt that traction assistance is key to facilitating esophageal ESD. Further studies are needed to elucidate the best method with respect to efficacy, safety, and cost.
Acknowledgments
I deeply thank Dr. Seung-Woo Lee (Division of Gastroenterology, Department of Internal Medicine, Daejeon St Mary’s Hospital, The Catholic University of Korea, Korea) and Minho Hwang (Department of Mechanical Engineering, Korea Advanced Institute of Science and Technology, Korea) for providing pictures of their research. I also thank L3 Inc. (https://www.l3japan.com/) for editing a draft of this manuscript.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Hon Chi Yip and Philip Wai-Yan Chiu) for the series “Endoscopic Diagnosis and Treatment of Early Esophageal Cancer” published in Annals of Esophagus. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://aoe.amegroups.com/article/view/10.21037/aoe-2020-34/coif). The series “Endoscopic Diagnosis and Treatment of Early Esophageal Cancer” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2015;47:829-54. [Crossref] [PubMed]
- Igaki H, Kato H, Tachimori Y, et al. Clinicopathologic characteristics and survival of patients with clinical Stage I squamous cell carcinomas of the thoracic esophagus treated with three-field lymph node dissection. Eur J Cardiothorac Surg 2001;20:1089-94. [Crossref] [PubMed]
- Inoue H, Endo M. Endoscopic esophageal mucosal resection using a transparent tube. Surg Endosc 1990;4:198-201. [Crossref] [PubMed]
- Inoue H, Takeshita K, Hori H, et al. Endoscopic mucosal resection with a cap-fitted panendoscope for esophagus, stomach, and colon mucosal lesions. Gastrointest Endosc 1993;39:58-62. [Crossref] [PubMed]
- Soetikno R, Kaltenbach T, Yeh R, et al. Endoscopic mucosal resection for early cancers of the upper gastrointestinal tract. J Clin Oncol 2005;23:4490-8. [Crossref] [PubMed]
- Ono H, Kondo H, Gotoda T, et al. Endoscopic mucosal resection for treatment of early gastric cancer. Gut 2001;48:225-9. [Crossref] [PubMed]
- Tanaka M, Ono H, Hasuike N, et al. Endoscopic submucosal dissection of early gastric cancer. Digestion 2008;77:23-8. [Crossref] [PubMed]
- Ono H, Hasuike N, Inui T, et al. Usefulness of a novel electrosurgical knife, the insulation-tipped diathermic knife-2, for endoscopic submucosal dissection of early gastric cancer. Gastric Cancer 2008;11:47-52. [Crossref] [PubMed]
- Kakushima N. Endoscopic submucosal dissection using the insulated-tip knife. Tech Gastrointest Endosc 2011;13:63-9.
- Lian J, Chen S, Zhang Y, et al. A meta-analysis of endoscopic submucosal dissection and EMR for early gastric cancer. Gastrointest Endosc 2012;76:763-70. [Crossref] [PubMed]
- Oyama T, Tomori A, Hotta K, et al. Endoscopic submucosal dissection of early esophageal cancer. Clin Gastroenterol Hepatol 2005;3:S67-70. [Crossref] [PubMed]
- Ono S, Fujishiro M, Niimi K, et al. Long-term outcomes of endoscopic submucosal dissection for superficial esophageal squamous cell neoplasms. Gastrointest Endosc 2009;70:860-6. [Crossref] [PubMed]
- Higuchi K, Tanabe S, Azuma M, et al. A phase II study of endoscopic submucosal dissection for superficial esophageal neoplasms (KDOG 0901). Gastrointest Endosc 2013;78:704-10. [Crossref] [PubMed]
- Yamashina T, Ishihara R, Nagai K, et al. Long-term outcome and metastatic risk after endoscopic resection of superficial esophageal squamous cell carcinoma. Am J Gastroenterol 2013;108:544-51. [Crossref] [PubMed]
- Tsujii Y, Nishida T, Nishiyama O, et al. Clinical outcomes of endoscopic submucosal dissection for superficial esophageal neoplasms: a multicenter retrospective cohort study. Endoscopy 2015;47:775-83. [Crossref] [PubMed]
- Minashi K, Nihei K, Mizusawa J, et al. Efficacy of Endoscopic Resection and Selective Chemoradiotherapy for Stage I Esophageal Squamous Cell Carcinoma. Gastroenterology 2019;157:382-390.e3. [Crossref] [PubMed]
- Fukami N. What we want for ESD is a second hand! Traction method. Gastrointest Endosc 2013;78:274-6. [Crossref] [PubMed]
- Oyama T. Counter traction makes endoscopic submucosal dissection easier. Clin Endosc 2012;45:375-8. [Crossref] [PubMed]
- Hirota M, Kato M, Yamasaki M, et al. A novel endoscopic submucosal dissection technique with robust and adjustable tissue traction. Endoscopy 2014;46:499-502. [Crossref] [PubMed]
- Ohata K, Fu K, Shouzushima M, et al. A novel traction system for esophageal endoscopic submucosal dissection. Endoscopy 2012;44 Suppl 2 UCTN:E410-1.
- Tsao SK, Toyonaga T, Morita Y, et al. Modified fishing-line traction system in endoscopic submucosal dissection of large esophageal tumors. Endoscopy 2011;43 Suppl 2 UCTN:E119.
- Chen PJ, Huang WC, Wang HP, et al. Percutaneous transgastric traction-assisted esophageal endoscopic submucosal dissection: a randomized controlled trial in a porcine model. Scand J Gastroenterol 2012;47:1386-93. [Crossref] [PubMed]
- Motohashi O, Nishimura K, Nakayama N, et al. Endoscopic submucosal dissection (two-point fixed ESD) for early esophageal cancer. Dig Endosc 2009;21:176-9. [Crossref] [PubMed]
- Ohata K, Fu K, Sakai E, et al. Esophageal Endoscopic Submucosal Dissection Assisted by an Overtube with a Traction Forceps: An Animal Study. Gastroenterol Res Pract 2016;2016:3186168. [Crossref] [PubMed]
- Zhang Q, Yao X, Cai JQ, et al. Snare combined with endoclips in endoscopic submucosal dissection with mucosal traction for gastroesophageal neoplasia. J Gastroenterol Hepatol 2019;34:1049-57. [Crossref] [PubMed]
- Suzuki S, Gotoda T, Kobayashi Y, et al. Usefulness of a traction method using dental floss and a hemoclip for gastric endoscopic submucosal dissection: a propensity score matching analysis (with videos). Gastrointest Endosc 2016;83:337-46. [Crossref] [PubMed]
- Yoshida M, Takizawa K, Ono H, et al. Efficacy of endoscopic submucosal dissection with dental floss clip traction for gastric epithelial neoplasia: a pilot study (with video). Surg Endosc 2016;30:3100-6. [Crossref] [PubMed]
- Yoshida M, Takizawa K, Suzuki S, et al. Conventional versus traction-assisted endoscopic submucosal dissection for gastric neoplasms: a multicenter, randomized controlled trial (with video). Gastrointest Endosc 2018;87:1231-40. [Crossref] [PubMed]
- Tsuji K, Yoshida N, Nakanishi H, et al. Recent traction methods for endoscopic submucosal dissection. World J Gastroenterol 2016;22:5917-26. [Crossref] [PubMed]
- Imaeda H, Hosoe N, Kashiwagi K, et al. Advanced endoscopic submucosal dissection with traction. World J Gastrointest Endosc 2014;6:286-95. [Crossref] [PubMed]
- Ota M, Nakamura T, Hayashi K, et al. Usefulness of clip traction in the early phase of esophageal endoscopic submucosal dissection. Dig Endosc 2012;24:315-8. [Crossref] [PubMed]
- Koike Y, Hirasawa D, Fujita N, et al. Usefulness of the thread-traction method in esophageal endoscopic submucosal dissection: randomized controlled trial. Dig Endosc 2015;27:303-9. [Crossref] [PubMed]
- Xie X, Bai JY, Fan CQ, et al. Application of clip traction in endoscopic submucosal dissection to the treatment of early esophageal carcinoma and precancerous lesions. Surg Endosc 2017;31:462-8. [Crossref] [PubMed]
- Yoshida M, Takizawa K, Nonaka S, et al. Conventional versus traction-assisted endoscopic submucosal dissection for large esophageal cancers: a multicenter, randomized controlled trial (with video). Gastrointest Endosc 2020;91:55-65.e2. [Crossref] [PubMed]
- Ishihara R, Iishi H, Takeuchi Y, et al. Local recurrence of large squamous-cell carcinoma of the esophagus after endoscopic resection. Gastrointest Endosc 2008;67:799-804. [Crossref] [PubMed]
- Ishihara R, Iishi H, Uedo N, et al. Comparison of EMR and endoscopic submucosal dissection for en bloc resection of early esophageal cancers in Japan. Gastrointest Endosc 2008;68:1066-72. [Crossref] [PubMed]
- von Delius S, Feussner H, Henke J, et al. Submucosal endoscopy: a novel approach to en bloc endoscopic mucosal resection (with videos). Gastrointest Endosc 2007;66:753-6. [Crossref] [PubMed]
- Huang R, Cai H, Zhao X, et al. Efficacy and safety of endoscopic submucosal tunnel dissection for superficial esophageal squamous cell carcinoma: a propensity score matching analysis. Gastrointest Endosc 2017;86:831-8. [Crossref] [PubMed]
- Zhang W, Zhai Y, Chai N, et al. Endoscopic submucosal tunnel dissection and endoscopic submucosal dissection for large superficial esophageal squamous cell neoplasm: efficacy and safety study to guide future practice. Surg Endosc 2018;32:2814-21. [Crossref] [PubMed]
- Abe S, Wu SYS, Ego M, et al. Efficacy of Current Traction Techniques for Endoscopic Submucosal Dissection. Gut Liver 2020;14:673-84. [Crossref] [PubMed]
- Li P, Ma B, Gong S, et al. Endoscopic submucosal tunnel dissection for superficial esophageal neoplastic lesions: a meta-analysis. Surg Endosc 2020;34:1214-23. [Crossref] [PubMed]
- Peng W, Tan S, Ren Y, et al. Efficacy and safety of endoscopic submucosal tunnel dissection for superficial esophageal neoplastic lesions: a systematic review and meta-analysis. J Cardiothorac Surg 2020;15:33. [Crossref] [PubMed]
- Jin P, Fu KI, Yu Y, et al. Traction using a clip-with-line is a preferred method for trainees in performing esophageal endoscopic submucosal dissection: an animal model study. Therap Adv Gastroenterol 2017;10:343-51. [Crossref] [PubMed]
- Jacques J, Legros R, Rivory J, et al. The "tunnel + clip" strategy standardised and facilitates oesophageal ESD procedures: a prospective, consecutive bi-centric study. Surg Endosc 2017;31:4838-47. [Crossref] [PubMed]
- Fraile-López M, Parra Blanco A. Double-tunnel circumferential endoscopic submucosal dissection with double clip-band-line traction for an esophageal squamous neoplasm. Endoscopy 2020;52:E303-5. [Crossref] [PubMed]
- Hwang M, Lee SW, Park KC, et al. Evaluation of a robotic arm-assisted endoscope to facilitate endoscopic submucosal dissection (with video). Gastrointest Endosc 2020;91:699-706. [Crossref] [PubMed]
Cite this article as: Yoshida M. Endoscopic submucosal dissection for large early squamous cell carcinoma—traction assisted methods. Ann Esophagus 2023;6:4.


