MIS revisional surgery for gastro-esophageal reflux disease: how I do it
Introduction
Minimally invasive surgery to control gastro-esophageal reflux disease (GERD) was introduced in 1991, and quickly became mainstream as an excellent option for patients with breakthrough symptoms on maximal medical therapy. However, a small subset of patients will be unhappy with the result of laparoscopic fundoplication (3–6%), and will present for consideration of a laparoscopic revisional procedure (1). Careful patient selection for laparoscopic revisional surgery is critical because revisional procedures carry a higher morbidity rate (23%), dysphagia rate (25%), and mortality rate (1%) (2).
In this paper, we discuss our definition of a failed fundoplication, and we outline our operative approach to a minimally invasive redo fundoplication. From open surgery (either via a thoracotomy or a laparotomy) to now laparoscopic surgery (sometimes with 3-dimensional cameras or robotic-assisted surgery), the approach to revisional fundoplication has evolved rapidly over the past two decades. With these advances, tips and tricks have been picked up and will be highlighted here. We also discuss our approach to postoperative management.
What is a failed fundoplication?
One of the biggest challenges in the management of GERD patients post fundoplication is that there is no accepted standard definition of what constitutes a failure of anti-reflux surgery. Failure can encompass all of the following: recurrent or residual reflux symptoms, use of anti-reflux medication, or de novo symptoms (including different reflux symptoms or side-effect symptoms from the procedure). Failure can be defined subjectively, through the patient’s description of their symptoms or objectively, through endoscopy, acidic pH or total reflux studies, esophageal manometry, and barium studies. A failed procedure may not necessarily have an identifiable anatomical cause. The International Society of the Diseases of the Esophagus (ISDE) is currently working on a document to further define the failed fundoplication—an essential step towards accurate reporting of failure from fundoplication, and the management of the failed fundoplication.
Our definition of a failed fundoplication
A laparoscopic fundoplication can often result in symptoms of bloating, increased flatulence, and dysphagia. These are side-effects of the procedure itself as circumferential swelling of the esophago-gastric junction (secondary to manipulation of the esophagus as well as the intra-operative use of an esophageal sling) can impede the passage of food into the stomach, and may result in a fundoplication which is air-tight as well as “acid-tight”. That said, these symptoms should subside once the postoperative swelling has subsided, usually within the first 3 months, and certainly by the 6-month mark. However, persistence of these symptoms, in particular dysphagia and bloating, for greater than 6 months is considered by many as a failed fundoplication.
The second group of patients with a failed fundoplication are those with recurrent reflux symptoms, often due to an anatomical problem in the peri-operative period. Common causes include a slipped wrap, a recurrent hiatus hernia, or perhaps the creation of a partial fundoplication where a total fundoplication should have been performed. This group of patients is relatively straightforward to manage as a laparoscopic revisional fundoplication should resolve the patient’s reflux symptoms.
The final group of patients with a failed fundoplication are those with residual reflux symptoms, i.e., their pre-operative symptoms continue to persist following surgery. These patients are often those with atypical reflux symptoms (i.e., cough, hoarseness, etc), or those with negative objective testing for GERD prior to surgical intervention. It is this group of patients whom the surgeon should try to avoid operating on in the first place, as this group often lack the correct indications for primary laparoscopic fundoplication, resulting in persistent symptoms and patient dissatisfaction.
Laparoscopic revisional surgery: technique
Patient selection
The most important first step for the surgeon is to take a thorough history. In particular, what was the indication for primary anti-reflux surgery? Further, was there objective evidence of reflux pre-operatively (either ulcerative esophagitis on endoscopy or a positive pH study)? The surgeon must not re-operate on a patient whose “recurrent reflux” is not true reflux (see “Choosing the right patient for laparoscopic fundoplication: a review of preoperative predictors” in this special edition). If the recurrent symptoms are different to those experienced by the patient pre-operatively, further investigations are warranted to exclude other causes such as biliary colic and peptic ulcer disease. Mandatory investigations when dealing with a presentation of recurrent reflux symptoms, include: endoscopy (ideally performed by the responsible surgeon), 24-hour pH study (if no evidence of reflux on endoscopy), esophageal manometry, and barium swallow (3).
Preoperative preparation
If the decision has been made to perform laparoscopic revisional surgery, optimize all conditions. All patients should commence a very low-calorie diet (VLCD) to shrink the liver, reduce visceral fat, and improve access to the hiatus (4). Most patients will require at least 2 weeks on a VLCD, whilst those with a BMI over 35 may benefit from at least 4 weeks. Enlist the assistance of an experienced second operator, and book adequate time for the procedure.
Positioning and trocar placement
The patient is positioned in the supine lithotomy (French) position to allow the surgeon to operate from between the legs. Steep reverse Trendelenburg positioning is then achieved with the patient on a gel mat. Consider port placement carefully. Do not reuse the old port sites if they are in the wrong place—it is best to optimize the field of view for the revisional procedure! We prefer a closed Veress technique in the left upper quadrant with insufflation to 10–14 mmHg. Port placement is as follows: 11 mm camera port 15 cm from the xiphoid, to the left of midline; 12 mm working port in the left upper quadrant 11 cm from the xiphoid along the costal margin; 5 mm working port in the right upper quadrant 11 cm from the xiphoid along the costal margin; and a 5 mm assistant port in the left lateral abdomen. A Nathanson liver retractor is placed at the level of the xiphoid.
It is a good idea to make sure the anesthetist has a 52- or 54-French bougie in the mid-esophagus to help with laparoscopic localisation of the esophagus. Primary anti-reflux surgery often displaces the esophagus to a more anterior location. An intra-operative endoscope may also be used instead of a bougie, depending on surgeon preference.
Operative technique—key steps
Our usual approach is to first restore normal anatomy. We start at the right pars flaccida and identify the right crus. We then carry the dissection across the anterior esophagus to the left crus. Be alert and prepared for braided sutures placed at the hiatus during primary laparoscopic fundoplication (e.g., Ethibond®), or mesh in situ. There will be far more scarring and fibrosis for braided sutures than for monofilament sutures used at primary operation. These patients should be made aware of the higher conversion rate to laparotomy for their revisional procedure. Consider the use cautery and ultrasonic shears judiciously around the esophagus. If the short gastric vessels were not divided at the first operation, this provides a nice uninterrupted plane to locate the left pillar of the crural diaphragm.
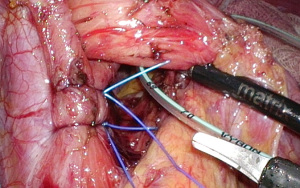
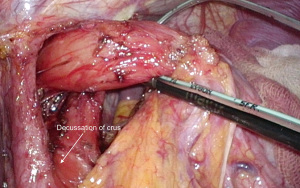
Once the normal anatomy has been restored, a sling (we prefer a pediatric 5-French feeding tube) is placed around the esophagus and care is taken to identify and preserve the posterior vagus. The right and left pillars of the diaphragm need to be identified clearly down to the crural decussation (Figure 1). The crural diaphragm is closed posteriorly with non-absorbable monofilament sutures (e.g., 2-0 NovafilTM), taking care not to angulate the esophagus (Figure 2). If this is a concern (i.e., the anesthetist cannot advance the bougie in a straight line), a combined posterior and anterior closure should be done. We use a 52-French or 54-French bougie to calibrate the diaphragmatic closure. With the bougie advanced into the stomach, there should be just enough space between the esophagus and the pillars to allow the easy passage of the tip of a laparoscopic grasper.
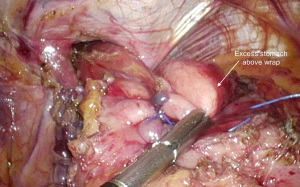
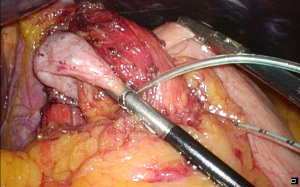
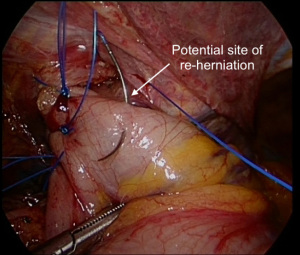
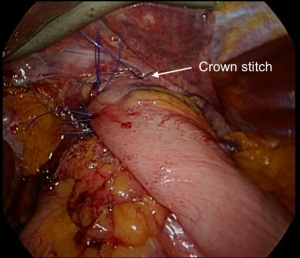
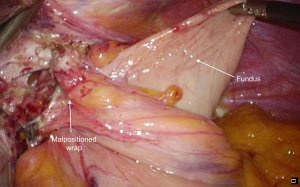
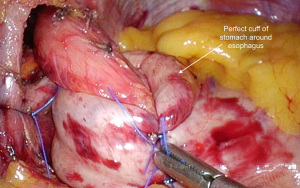
A fundoplication is then re-created, ensuring there is adequate intra-abdominal esophageal length. We believe that with adequate peri-esophageal dissection 5–6 cm up into the chest, cases of true shortened esophagus are rare. With many revisional cases, the cause of a slipped wrap is due to misidentification of the fundus of the stomach at primary surgery (Figure 3). Always ensure the top-most aspect of the stomach is used to create the fundoplication, whether for a partial or total fundoplication. For a Nissen fundoplication, avoid excess stomach above the wrap (Figure 4). The key is a shoe-shine manoeuvre (Figure 5) which avoids inadvertent excess stomach posterior to the esophagus (Figure 6). We prefer to calibrate a total fundoplication over a 54-French bougie. It is important to recognize that if the short gastric vessels have not been divided, the Nissen fundoplication will face towards the patient’s caudate lobe. For a partial anterior 180-degree fundoplication, the most common site of re-herniation of the gastric fundus into the mediastinum occurs at the 1 or 2 o’clock position (Figure 7). Consider the insertion of a crown stitch (Figure 8).
Postoperative care
Post-operative care should focus on early mobilization and avoidance of nausea. We prefer to use Ondansetron or Tropisetron routinely for the first 24–48 hours post-surgery. After 12–24 hours, no pain relief should be necessary aside from paracetamol. Our institutional protocol is to perform a contrast swallow the following morning. This provides confirmation of correct positioning of the fundoplication below the diaphragm, and confirms the absence of a leak. We have recently shown that routine postoperative contrast swallows, primarily in patients with a hiatus hernia, reduces the morbidity related to early and late reoperations (5). Once the swallow has been performed and reviewed, the patients start a fluid diet and are discharged home on puréed/vitamised food for 1 to 2 weeks. They then continue onto a soft diet for a further 4 weeks. Care is taken to avoid constipation in the early post-operative period and our patients are counselled to take a stool softener for the first couple of weeks if needed.
Conclusions
As highlighted above, the International Society of the Diseases of the Esophagus (ISDE) is currently working on a document to define the failed fundoplication—an essential step towards accurate reporting of failure from fundoplication, and the management of the failed fundoplication. We believe there are three categories of patients with a “failed fundoplication”: (I) patients with troublesome and persistent side-effects of a laparoscopic fundoplication. Most commonly, this includes bloating, increased flatulence, and dysphagia; (II) patients with recurrent reflux symptoms, often due to an anatomical problem such as a slipped wrap, a recurrent hiatus hernia, or a mal-positioned fundoplication; (III) patients with residual reflux symptoms, often those with atypical symptoms that may not have been due to reflux in the first place (e.g., cough).
Laparoscopic revisional fundoplication is indicated for patients with true recurrent reflux, as well as those with distressing complications of surgery including dysphagia and bloating. Of utmost importance is patient selection, both at the primary and revisional procedure. It is wise not to assume the correct indication was present at the initial procedure, and prudent to fully investigate any patient with recurrent symptoms.
Thorough investigation with endoscopy, barium swallow, pH testing, and esophageal manometry is advisable prior to laparoscopic revisional surgery. Our key steps are highlighted above, and our postoperative management including routine anti-emetics, postoperative contrast swallow, and careful dietary counselling. With these steps, 86% of patients undergoing a laparoscopic revisional fundoplication in our institution are satisfied or highly satisfied with the result (6).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Timothy M. Farrell and Geoffrey Kohn) for the series “Minimally Invasive Procedures for Gastroesophageal Reflux Disease” published in Annals of Esophagus. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-26/coif). The series “Minimally Invasive Procedures for Gastroesophageal Reflux Disease” was commissioned by the editorial office without any funding or sponsorship. SKT serves as an unpaid editorial board member of Annals of Esophagus from September 2019 to August 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Carlson MA, Frantzides CT. Complications and results of primary minimally invasive antireflux procedures: a review of 10,735 reported cases. J Am Coll Surg 2001;193:428-39. [Crossref] [PubMed]
- Yadlapati R, Hungness ES, Pandolfino JE. Complications of Antireflux Surgery. Am J Gastroenterol 2018;113:1137-47. [Crossref] [PubMed]
- Allaix ME, Rebecchi F, Schlottmann F, et al. Secrets for successful laparoscopic antireflux surgery: adequate follow-up. Ann Laparosc Endosc Surg 2017;2:57-60. [Crossref]
- Lewis MC, Phillips ML, Slavotinek JP, et al. Change in liver size and fat content after treatment with Optifast very low calorie diet. Obes Surg 2006;16:697-701. [Crossref] [PubMed]
- Liu DS, Wee MY, Grantham J, et al. Routine esophagograms after hiatus hernia repair minimizes reoperative morbidity: a multicenter comparative cohort study. Ann Surg 2022;276:e770-6. [Crossref] [PubMed]
- Lamb PJ, Myers JC, Jamieson GG, et al. Long-term outcomes of revisional surgery following laparoscopic fundoplication. Br J Surg 2009;96:391-7. [Crossref] [PubMed]
Cite this article as: Thompson SK, Shukla RN. MIS revisional surgery for gastro-esophageal reflux disease: how I do it. Ann Esophagus 2022;5:40.