
Pre-operative evaluation of gastro-esophageal reflux disease
Introduction
Surgery is a valid therapeutic option for gastro-esophageal reflux disease (GERD). The classical anti-reflux procedure is a fundoplication usually performed laparoscopically. A pre-operative work up is necessary to select patients for surgery and to eliminate conditions that could contra-indicate surgery.
Currently patients with typical GERD symptoms (heartburn, regurgitation) and good response to proton pump inhibitors (PPI) are good candidates for anti-reflux surgery (1). However, even in this group of patients, the 5-year outcome after fundoplication is not better than the outcome on PPI therapy (2). The caveat is for patients with prominent regurgitation who seem to benefit more from surgery than from medical treatment. Thus, the clinical evaluation is an important step in patients’ selection for surgery. In some instances, a non-response or a partial response to PPI could be as well an indication for surgery. A recent prospective randomized trial demonstrated that some patients with persistent typical symptoms and proven reflux on PPI might have better outcome after surgery than on medical therapy combining PPI and reflux inhibitors (baclofen) (3). The occurrence of atypical symptoms remains a difficult situation and caution is warranted before offering anti-reflux surgery. Even if an intensive work-up links a typical symptom to reflux episodes, it does not reliably predict good response to surgery. Therefore, patient selection for surgery is crucial. Symptom presentation alone (typical or atypical symptoms) is not sufficient to indicate surgery and the confirmation of GERD diagnosis (as will be detailed below) is essential.
Besides the confirmation of GERD diagnosis, it is important to rule out contra-indication to surgical treatment and to search for predictive factors of response and/or side effects. For example, the occurrence of esophageal motility disorders can contra-indicate surgery or lead to a modification of surgical procedure (partial rather than complete fundoplication).
Guidelines are available to select patients for anti-reflux surgery (ICARUS, international consensus regarding preoperative examinations and clinical characteristics assessment to select adult patients for antireflux surgery) (1). The minimal pre-operative work up consists usually of upper gastro-intestinal (GI) endoscopy and pH-(impedance) monitoring (if necessary) to confirm the diagnosis of GERD, and of esophageal manometry to rule out esophageal motility disorders. Radiological examination (and in particular esophagogram) is frequently performed to describe esophago-gastric junction anatomy.
This review on pre-operative evaluation of GERD summarizes the procedures and the results of the examinations performed to confirm the diagnosis of GERD and evaluation esophageal function and anatomy.
Confirmation of the diagnosis of GERD
The probability of GERD is higher in patients with typical symptoms than in those with atypical symptoms. Nevertheless, clinical symptoms alone are not sufficient to confirm the diagnosis of GERD when surgical treatment is considered. Different examinations are thus required to confirm the diagnosis of GERD and to establish a relationship between symptoms and reflux episodes.
Upper GI endoscopy
Upper GI endoscopy is the first line examination in patients with GERD symptoms. The indications are an age older than 50 years, the occurrence of alarm signs (weight loss, bleeding, anemia, dysphagia, odynophagia), persistent or recurrent symptoms despite PPI treatment, previous family history of cancer and/or when surgical treatment is considered. ICARUS guidelines mentions that endoscopy is mandatory in the last year prior to anti-reflux surgery and there is no need to perform this examination off PPI in the pre-operative work up (1). The aim of this examination is to rule out esophageal or gastric lesions (for example esophageal tumor) that may explain symptoms and to search for mucosal complications related to GERD. The assessment of Barrett’s esophagus (and dysplasia) is important in order to organize patient’s follow up and treatment. The identification of hiatal hernia (size, configuration) and short esophagus may help the surgeon to choose the anti-reflux procedure. Moreover, patients with hiatal hernia and GERD symptoms are good candidates for anti-reflux surgery (1).
The Lyon Consensus defined pathological GERD on upper GI endoscopy as the occurrence of Los Angeles grade C or D esophagitis, Barrett’s mucosa greater than 1 cm or peptic stricture (4). Grade A esophagitis (defined as mucosal breaks no longer than 5 mm) can be encountered in asymptomatic subjects (5) and therefore is not sufficient for the diagnosis of GERD. According to the Lyon consensus, grade B esophagitis was not sufficient as well to confirm the diagnosis of GERD when a surgical treatment was considered. While grade B esophagitis is rarely shown in asymptomatic controls, progression from grade A/B esophagitis to grade C/D esophagitis is encountered in only 1% to 6% of cases and progression from grade A/B to Barrett’s esophagus in 1% to 12% (6). Further grade A/B esophagitis may regress to normal endoscopy in 20% to 60%. Due to this natural history, the question of including grade B esophagitis as robust sign of GERD was debated during the Lyon Consensus and the experts recommended the confirmation of GERD diagnosis with reflux monitoring before referring a patient with grade B esophagitis to surgery.
Upper GI endoscopy can also identify a hiatal hernia that is a risk factor for GERD. However, hiatal hernia is not synonymous of GERD and the indication of anti-reflux surgery cannot rely only on a finding of hiatal hernia on endoscopy.
Overall, endoscopic abnormalities are present in 10% to 30% of patients with GERD. Thus, it is frequently necessary to perform reflux monitoring to confirm the diagnosis of GERD (see below). Some authors have proposed to use esophageal biopsies to improve the diagnosis of endoscopy. Unfortunately, microscopic esophagitis has a limited diagnostic value for the diagnosis of GERD and the indication of anti-reflux surgery cannot rely on this finding. Performing esophageal biopsies can be of interest to depict eosinophilic esophagitis. An overlap between GERD, eosinophilic esophagitis and PPI-responsive eosinophilia is now admitted but patients with eosinophilic esophagitis are poor candidates to anti-reflux surgery (1). It is important to note that the prevalence of eosinophilic esophagitis seems to be low in adults with refractory heartburn. Therefore, obtaining esophageal biopsies in all patients evaluated for anti-reflux surgery is debatable.
Reflux monitoring
Reflux monitoring allows the identification of reflux episodes into the esophagus. It is considered as the gold standard for the diagnosis of GERD. However, this examination is not perfect as pathological reflux can be intermittent and cannot occur during the monitoring period.
Three methods are available to detect reflux episodes into the esophagus: catheter-based pH-monitoring, wireless pH-monitoring, and pH-impedance monitoring (this latter one is catheter-based technique). pH-monitoring detects reflux episodes as the presence of acid into the esophagus (reflux being defined as an esophageal pH <4) while impedance identifies reflux as the anterograde propagation of liquid (+/− air) into the esophagus. Thus only acid reflux episodes are detected with catheter-based and wireless pH monitoring. With pH-impedance monitoring it is possible to identify not only acid but also weakly acid reflux episodes as the detection is based on the presence of liquid into the esophagus. Reflux monitoring is performed ambulatory during 24 hours for catheter-based pH-(impedance) monitoring and during 48 to 96 hours for wireless pH-monitoring.
According to the Lyon consensus, in a patient without proven GERD (no grade C or D esophagitis, no Barrett’s mucosa, no peptic stricture), reflux detection should be performed off PPI with one of these three techniques. The wireless pH-monitoring has the advantages of a better tolerance with fewer dietary modifications and restrictions on activities compared to catheter-based system and prolonged recording (up to 96 hours) (7). Thus it is more sensitive than other techniques for reflux detection. However, it is not available everywhere and not reimbursed in some countries.
In a patient with proven GERD (grade C or D esophagitis, Barrett’s esophagus >1 cm, peptic stricture) and persistent symptoms on PPI, reflux detection should be performed on PPI using pH-impedance monitoring. Indeed, in this subset of patients the question is to know if residual symptoms are related to persistent reflux on PPI and the majority of reflux episodes are weakly acidic in a patient on PPI.
Based on the Lyon consensus, the best parameter to confirm the diagnosis of GERD on reflux monitoring is the esophageal acid exposure time (AET). AET is pathological if is greater than 6% of the total time and normal if lower than 4%. In between, there is a grey area in which the diagnosis of GERD is possible but additional parameters are required. One of these parameters is the number of reflux episodes (pathological than 80/24 h on impedance monitoring). The Lyon consensus proposed to use the same threshold for studies performed off or on PPI. Some recent data in a large international cohort of normal subjects suggested that lower thresholds could be used for the diagnosis of pathological GERD with pH-impedance monitoring (8). Another important finding from this international cohort is that thresholds might differ from one country to another. Other parameters such mean nocturnal baseline impedance (MNBI) or post reflux swallow-induced peristaltic wave (PSPW) were considered as exploratory tools in the Lyon Consensus. Indeed, there are some data (mainly retrospective) to suggest that these parameters could predict response to GERD treatment. However, the experts thought that further studies would be required to confirm these promising results.
The relationship between reflux episodes and symptom can be evaluated during reflux monitoring. The patient can report symptom by pushing a button on the recorder and/or filling a diary. A symptom event occurring within the 2 minutes following the reflux episode is considered to be secondary to reflux. According to the Lyon consensus, to be reliable, the analysis of reflux-symptom association should be based on at least three symptom events reported by the patient. Further, this analysis is possible for symptom with a precise onset such as heartburn, regurgitation, chest pain or cough. This is more difficult to assess the relationship when patient reports atypical symptoms such as sore throat or dental erosion. Two indices are available to assess the association reflux-symptom: the symptom index (SI) and the symptom association probability (SAP). SI is the percentage of symptom episodes that correlate with reflux episodes. A SI >50% is considered as positive. A positive SI is a predictive factor of good response to PPI therapy and to surgery. The SAP assesses the likelihood that patient’s symptoms are related to reflux. This is a statistical test (Fisher’s exact test). SAP is a predictor of good response to anti-reflux surgery. Having both SI and SAP positive seems to be more robust than one single positive index to establish the relationship between reflux episodes and symptoms events (4).
Indications of anti-reflux surgery
Patients with GERD symptoms and grade C or D esophagitis are good candidates for surgery. The presence of GERD symptoms and hiatal hernia or grade B esophagitis were considered as good candidates as by the experts of the ICARUS guidelines (1). These experts recommended esophageal pH-(impedance) monitoring off therapy before anti-reflux surgery to confirm the diagnosis of GERD in patients without esophagitis (1). The recommendation included as well patients with short Barrett’s esophagus in absence of erosive esophagitis.
Based on pH-(impedance) monitoring, the indications of anti-reflux surgery were discussed in the ICARUS guidelines. Few statements achieved a consensus (1). The expert agreed on the fact that patients with normal pH-monitoring off PPI were poor candidates to anti-reflux surgery. Pathological acid exposure is generally admitted to be a good indication of surgery as long as the patients have typical symptoms and response to PPI therapy. Recent data suggest that the number of reflux episodes detected on pH-impedance monitoring can be predictive on outcome in patients with regurgitation (9). So it might be of interest to discuss surgery in these patients especially if there are other arguments in favor of surgery (clinical presentation, hiatal hernia…). A positive symptom-reflux association in absence of abnormal reflux on pH-(impedance) monitoring is not sufficient to indicate anti-reflux surgery. Recently, a large randomized trial explored the yield of anti-reflux surgery in patients with typical GERD symptoms who did not respond to PPI therapy (3). In highly selected patients with proven GERD off PPI, persistent heartburn and pathological reflux on pH-impedance monitoring, anti-reflux surgery might be associated with better outcome than medical treatment. It is important to note that 366 patients were enrolled on symptoms presentation (refractory GERD symptoms) but only 78 (21%) were randomized after the work-up. Indeed, despite symptoms on PPI at enrollment, some patients improved on a well-conducted PPI therapy and other did have not persistent pathological GERD on examinations. This study emphasizes the role of an extensive work up before referring patients for anti-surgery.
Evaluation of esophageal motility
Esophageal manometry is mandatory before anti-reflux surgery (1). The aim of this examination is to rule out major motility disorders that could contra-indicate surgery. The superiority of high resolution manometry (HRM) over conventional manometry was demonstrated in particular for the diagnosis of achalasia (10). There is no data to confirm HRM superiority in patients referred for manometry before anti-surgery. However, it is important to note that HRM replaces progressively conventional manometry and the diagnosis yield demonstrated for patients with dysphagia might exist for other indications as well.
Achalasia is a contra-indication to anti-reflux surgery (unless the fundoplication is performed in combination with a Heller myotomy). In a series of 1,081 patients referred for esophageal manometry before anti-reflux surgery, 1% of them had achalasia (11). Indeed regurgitation, heartburn, and/or chest pain might be the main symptoms in patients with achalasia without obvious dysphagia. Ruling out achalasia with manometry is thus essential in patients referred for anti-reflux surgery.
Absent contractility and esophago-gastric junction outflow obstruction are also considered as contra-indications to anti-reflux (1). Some questions are raised for these two types of motility disorders. The first one concerns scleroderma which is associated with GERD in a large majority of patients. Absent esophageal contractility is a hallmark of esophageal involvement in scleroderma. As GERD can be severe in scleroderma, some authors have proposed anti-reflux surgery despite the absence of esophageal contractility. Outcome data are contradictory and no consensus was achieved to contra-indicate systematically anti-reflux surgery in scleroderma patients in the ICARUS guidelines (1). A case-based discussion is recommended in these patients if they have severe GERD symptoms and/or esophagitis despite PPI therapy. Alternative surgery such as Roux-en-Y gastric bypass might be an option in well-selected patients with scleroderma. Regarding EGJ outflow obstruction, the problem is the clinical significant of this motility disorder. This is now addressed in the latest version of the Chicago Classification (12). The manometric diagnosis of EGJ outflow obstruction (requiring an abnormal EGJ relaxation in supine and upright position and an elevated intra-bolus pressure without criteria for achalasia) is always considered clinically inconclusive in the Chicago Classification v4.0. Supportive investigations (barium esophagogram, EndoFLIP™) are required to confirm the clinical relevance in patients with dysphagia and/or non cardiac chest pain and manometric pattern of EGJ outflow obstruction. If the clinical relevance of EGJ outflow obstruction is confirmed, the contra-indication of anti-reflux surgery is logical.
There are no data to determine if hypercontractile esophagus or distal esophageal spasm are risk factor for dysphagia after fundoplication. However, caution could be recommended in these patients and some complementary examinations to asses for example esophageal clearance could be helpful to evaluate the clinical relevance of these manometric patterns in particular if the patient has no dysphagia.
Hypotensive esophageal motility disorders are frequently encountered in GERD (Figure 1) (13). As the fundoplication restores the anti-reflux and could thus increase the pressure at the level of the EGJ, there is a concern that pre-existing esophageal hypomotility could be associated with post operative dysphagia. In a series of 68 patients who underwent pre and post anti-reflux surgery HRM, the authors observed four phenotypes: a persistent esophageal hypomotility after surgery (15%), a resolved hypomotility after surgery (9%), a newly developed hypomotility after surgery (19%) and the absence of hypomotility both before and after surgery (57%) (14). Thus anti-reflux can induce esophageal motility disorders but can also resolve these disorders. Therefore, hypomotility should not be considered as a strict contra-indication to anti-reflux. The risk of post-operative dysphagia depends on the type of surgery. It is higher after Nissen fundoplication (360°) than after Toupet (270°) (15). Fibbe et al. explored the interest of tailoring anti-reflux surgery according to esophageal motility disorders (16). Patients were stratified according to pre-operative motility disorders and randomized to Nissen or Toupet fundoplication. While pre-operative esophageal dysmotility was associated with more severe reflux symptoms, clinical outcome and reflux recurrence were similar and not associated with pre-operative motility disorders. The authors concluding that tailoring surgical management was not required. This study was performed with conventional manometry. At the era of HRM, it is unknown if this conclusion is still true.
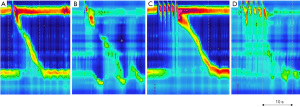
Importantly some authors have proposed to perform provocative maneuvers during pre-operative HRM to determine the risk of post-operative dysphagia. In a retrospective study, Shaker et al. demonstrated that response to multiple rapid swallows (MRS) on pre-operative HRM predicted late post-operative dysphagia. MRS consists of five-2 mL water swallows performed in rapid succession (less than 4-s interval between swallows). MRS is considered as normal if the esophageal contraction that follows the 5th swallow is more vigorous than the esophageal contractions observed after single 5-mL water swallows (Figure 1). In a series of 63 patients who underwent anti-reflux surgery, normal response to response was observed in 64% of patients without post-operative dysphagia versus 11% of patients with late post-operative dysphagia (17). Thus, the absence of response after MRS could be predict post-operative dysphagia. MRS is easy to perform and could be helpful to predict post-operative dysphagia in particular in patient with esophageal hypomotility. Further prospective studies are required to confirm the role of MRS to predict post-operative dysphagia.
Incorporating HRM and impedance measurement might be useful in these cases of hypomotility to evaluate the risk of post-operative dysphagia. Indeed, some authors have developed a dysphagia risk index, including pressure and impedance parameters (time from nadir esophageal impedance to peak esophageal pressure, intra-bolus pressure, and rate of bolus pressure rise) (18). To develop this index they included 19 patients with pre and post-operative impedance-combined HRM. Five months after surgery, seven patients complained of new onset dysphagia. Using ROC analysis, the authors concluded that a dysphagia risk index >14 was optimally predictive of dysphagia with a sensitivity of 75% and a specificity of 93%. This index is promising to select patients before anti-reflux surgery. Prospective studies to confirm the role of this index are lacking so far.
Finally, HRM allows not only the assessment of esophageal motility but also the analysis of the morphology and the pressure of the EGJ. One study demonstrated that HRM was accurate for the diagnosis of hiatal hernia (19). Separation between lower esophageal sphincter and crural diaphragm on HRM is clearly associated with pathological reflux (20). EGJ is also less vigorous is patients with GERD compared to those without (21). Therefore, the EGJ findings on HRM can be an adjunctive tool for the diagnosis of GERD (4). It remains to determine if these findings can help to select patients for surgery.
Other examinations
Barium esophagogram
Hiatal hernia might represent a good indication of anti-reflux in patients with GERD symptoms. Therefore, evaluating the esophago-gastric anatomy could be of interest to identify hiatal hernia that is an argument in favor of surgery. Barium esophagogram is superior to endoscopy for the diagnosis of hiatal hernia.
Further, according to the ICARUS guidelines, in patients with suspicion of hiatal hernia or short esophagus, barium esophagogram is mandatory in the preoperative workup for anti-reflux surgery (1). Recognizing a shortened esophagus before surgery may modify the choice of antireflux procedure. The signs in favor of a shortened esophagus are a hiatal hernia greater than 5 cm, a straightening of a loss of the angle of His, a stricture or a type III mixed or complex para-esophageal hernia.
Endoscopic functional lumen imaging probe (EndoFLIP™)
EndoFLIP™ has recently demonstrated its role to evaluate EGJ and esophageal distensibility (22). In patients with GERD, preliminary studies demonstrated an increased distensibility of the EGJ and the possibility to identify hiatal hernia. It has been used as well to tailor fundoplication (23). Fundoplication is associated with a decreased of EGJ distensbility but post-operative studies are controversial concerning the correlation between symptoms and EndoFLIP™ findings (22). The role of EndoFLIP™ in the pre-operative work up remains to be determined.
Evaluation of gastric emptying
Delayed gastric emptying might be a risk factor for GERD. Anti-reflux surgery can affect gastric emptying and gas bloat syndrome is one side effect of this surgery. However, there is no data to support that delayed gastric emptying can predict the occurrence of anti-reflux surgery complications. Therefore, according to the ICARUS guidelines, there is no need to assess gastric emptying rate in the pre-operative work up before anti-reflux surgery (1).
Conclusions
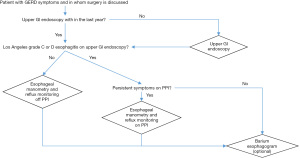
Selecting patients for anti-reflux surgery is crucial. The clinical presentation is important but not sufficient to indicate surgery. Therefore, complementary examinations are mandatory to confirm the diagnosis of GERD and to search for contra-indications. According to the ICARUS guidelines, upper GI endoscopy, reflux monitoring and esophageal manometry are required (1). Barium esophagogram can also be of interest to describe the esophago-gastric anatomy and orientate the choice of anti-reflux surgery. Figure 2 proposes an algorithm to organize pre-operative work up when surgery is considered in a patient with GERD symptoms. In absence of severe esophagitis, reflux monitoring is essential to select good candidates to anti-reflux surgery who are symptomatic patients with abnormal acid esophageal exposure off PPI. Persistent symptoms on PPI can be also an indication for surgery as long as pathological GERD is confirmed on pH-impedance monitoring performed on PPI. Esophageal manometry is mandatory to search motility disorders that could contra-indicate surgery but there are no data to confirm that manometry is useful to tailor the anti-reflux procedure. Prospective data are required to determine if the generalization of HRM could be helpful to choose the best anti-reflux surgery according to the esophageal motility findings.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Timothy M. Farrell and Geoffrey Kohn) for the series “Minimally Invasive Procedures for Gastroesophageal Reflux Disease” published in Annals of Esophagus. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-9/coif). The series “Minimally Invasive Procedures for Gastroesophageal Reflux Disease” was commissioned by the editorial office without any funding or sponsorship. The author reports Research support from Medtronic and Diversatek Healthcare and Consulting for Reckitt Benckiser. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pauwels A, Boecxstaens V, Andrews CN, et al. How to select patients for antireflux surgery? The ICARUS guidelines (international consensus regarding preoperative examinations and clinical characteristics assessment to select adult patients for antireflux surgery). Gut 2019;68:1928-41. [Crossref] [PubMed]
- Galmiche JP, Hatlebakk J, Attwood S, et al. Laparoscopic antireflux surgery vs esomeprazole treatment for chronic GERD: the LOTUS randomized clinical trial. JAMA 2011;305:1969-77. [Crossref] [PubMed]
- Spechler SJ, Hunter JG, Jones KM, et al. Randomized Trial of Medical versus Surgical Treatment for Refractory Heartburn. N Engl J Med 2019;381:1513-23. [Crossref] [PubMed]
- Gyawali CP, Kahrilas PJ, Savarino E, et al. Modern diagnosis of GERD: the Lyon Consensus. Gut 2018;67:1351-62. [Crossref] [PubMed]
- Ronkainen J, Aro P, Storskrubb T, et al. High prevalence of gastroesophageal reflux symptoms and esophagitis with or without symptoms in the general adult Swedish population: a Kalixanda study report. Scand J Gastroenterol 2005;40:275-85. [Crossref] [PubMed]
- Savarino E, de Bortoli N, De Cassan C, et al. The natural history of gastro-esophageal reflux disease: a comprehensive review. Dis Esophagus 2017;30:1-9. [PubMed]
- Roman S, Mion F, Zerbib F, et al. Wireless pH capsule--yield in clinical practice. Endoscopy 2012;44:270-6. [Crossref] [PubMed]
- Sifrim D, Roman S, Savarino E, et al. Normal values and regional differences in oesophageal impedance-pH metrics: a consensus analysis of impedance-pH studies from around the world. Gut 2020;gutjnl-2020-322627.
- Rogers BD, Valdovinos LR, Crowell MD, et al. Number of reflux episodes on pH-impedance monitoring associates with improved symptom outcome and treatment satisfaction in gastro-oesophageal reflux disease (GERD) patients with regurgitation. Gut 2021;70:450-5. [Crossref] [PubMed]
- Roman S, Huot L, Zerbib F, et al. High-Resolution Manometry Improves the Diagnosis of Esophageal Motility Disorders in Patients With Dysphagia: A Randomized Multicenter Study. Am J Gastroenterol 2016;111:372-80. [Crossref] [PubMed]
- Chan WW, Haroian LR, Gyawali CP. Value of preoperative esophageal function studies before laparoscopic antireflux surgery. Surg Endosc 2011;25:2943-9. [Crossref] [PubMed]
- Yadlapati R, Kahrilas PJ, Fox MR, et al. Esophageal motility disorders on high-resolution manometry: Chicago classification version 4.0© Neurogastroenterol Motil 2021;33:e14058. [Crossref] [PubMed]
- Gyawali CP, Roman S, Bredenoord AJ, et al. Classification of esophageal motor findings in gastro-esophageal reflux disease: Conclusions from an international consensus group. Neurogastroenterol Motil 2017;29. [Crossref] [PubMed]
- Mello MD, Shriver AR, Li Y, et al. Ineffective esophageal motility phenotypes following fundoplication in gastroesophageal reflux disease. Neurogastroenterol Motil 2016;28:292-8. [Crossref] [PubMed]
- Strate U, Emmermann A, Fibbe C, et al. Laparoscopic fundoplication: Nissen versus Toupet two-year outcome of a prospective randomized study of 200 patients regarding preoperative esophageal motility. Surg Endosc 2008;22:21-30. [Crossref] [PubMed]
- Fibbe C, Layer P, Keller J, et al. Esophageal motility in reflux disease before and after fundoplication: a prospective, randomized, clinical, and manometric study. Gastroenterology 2001;121:5-14. [Crossref] [PubMed]
- Shaker A, Stoikes N, Drapekin J, et al. Multiple rapid swallow responses during esophageal high-resolution manometry reflect esophageal body peristaltic reserve. Am J Gastroenterol 2013;108:1706-12. [Crossref] [PubMed]
- Myers JC, Nguyen NQ, Jamieson GG, et al. Susceptibility to dysphagia after fundoplication revealed by novel automated impedance manometry analysis. Neurogastroenterol Motil 2012;24:812-e393. [Crossref] [PubMed]
- Weijenborg PW, van Hoeij FB, Smout AJ, et al. Accuracy of hiatal hernia detection with esophageal high-resolution manometry. Neurogastroenterol Motil 2015;27:293-9. [Crossref] [PubMed]
- Tolone S, de Cassan C, de Bortoli N, et al. Esophagogastric junction morphology is associated with a positive impedance-pH monitoring in patients with GERD. Neurogastroenterol Motil 2015;27:1175-82. [Crossref] [PubMed]
- Tolone S, De Bortoli N, Marabotto E, et al. Esophagogastric junction contractility for clinical assessment in patients with GERD: a real added value? Neurogastroenterol Motil 2015;27:1423-31. [Crossref] [PubMed]
- Savarino E, di Pietro M, Bredenoord AJ, et al. Use of the Functional Lumen Imaging Probe in Clinical Esophagology. Am J Gastroenterol 2020;115:1786-96. [Crossref] [PubMed]
- Desprez C, Roman S, Leroi AM, et al. The use of impedance planimetry (Endoscopic Functional Lumen Imaging Probe, EndoFLIP(®)) in the gastrointestinal tract: A systematic review. Neurogastroenterol Motil 2020;32:e13980. [Crossref] [PubMed]
Cite this article as: Roman S. Pre-operative evaluation of gastro-esophageal reflux disease. Ann Esophagus 2022;5:36.


