
Endoscopic management of complications—endovacuum for management of anastomotic leakages: a narrative review
Introduction
Esophagectomy with gastric pull up and intrathoracic or cervical anastomosis is a standard part of treatment for esophageal cancer (1). Further indications include benign tumors and esophageal perforation. Most surgical approaches include either intrathoracic (Ivor-Lewis-procedure) or intracervical (McKeown-procedure) anastomoses (2,3). These anastomotic sites are susceptible to postoperative complications like leakages, bleedings, stricture or fistula (4). Thereby, anastomotic leakage remains the most common, yet threatening complication, with reported occurrence rates of 2–25% (5-8). Particularly intrathoracic leakages can cause severe mediastinitis and sepsis. Anastomotic leakage leads to prolonged ICU stay, hospital stay, high postoperative mortality and reduced quality of life (9). Additionally, potential treatment complications like anastomotic stenosis and stricture add up to these consequences. Reported risk factors for anastomotic leakages include prior radiation, technical errors, cervical location of the anastomosis and comorbidities as diabetes, active smoking, malnutrition, corticosteroid usage, and atherosclerotic calcification of the aorta (10,11).
As for leakage therapy, surgery is usually only performed in very early postoperative leakage, after failure of conservative treatments or acute unstable patients, given the comparable high mortality rates after reoperation. Nowadays different conservative options are applicable. Indeed, high-volume centers reach lower mortality rates not only by prevention, but also by optimized complication management (12). However, optimal treatment strategy remains unclear. Endoluminal stenting with a self-expanding-metal stent (SEMS) has become standard therapy in recent years, reaching successful healing rates up to 80–85% according to the literature (13). Limitations remain threatening post-interventional complications such as stent migration, defect enlargement and fistula formation, as well as the unsolved problem of sufficient wound drainage. Patients with mediastinitis need additional drainage therapy to relieve potential septic focus.
Most recently, the endoscopic vacuum therapy (EVAC) has been implemented as an innovative endoscopic treatment. Thereby, an open-pore polyurethane sponge with applied suction leading to negative pressure at the defective area is placed endoscopically. First articles of successful treatment with EVAC in the upper Gastrointestinal (GI) tract were published in 2008 (14), followed by several case series and studies reporting a treatment success rate of more than 80%. Although some cohort studies seem to prove superior effectiveness of EVAC compared to SEMS-therapy (15), no clear evidence supported by prospective and randomized studies is available so far (8). We present the following article in accordance with the Narrative Review reporting checklist (available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-16/rc).
Mechanism of EVAC therapy
The idea of the EVAC therapy derived from the well-established vacuum assisted closure therapy (VAC) used for the care of superficial wounds (16). The endoluminal mechanisms treating transmural defects follow the same principles. First endoluminal vacuum therapies were applied rectally for leakage after rectum resection, later the same principle was applied for the upper GI tract (17).
Exudate and infect control
A great part of its efficiency treating anastomotic leakage derives from its ability to drain fluid that forms in and around the defect (18,19). Hence, additional drains put in interventionally are not necessary. The accumulation of fluid intrathoracically and around the defect hinders healing by pressuring local cells and tissue. Some studies also imply local bacterial clearance and reduction of bacterial load to improve healing (20).
Macrodeformation and microdeformation
When suction is applied, two main changes occur to the affected tissue: macro- and microdeformation. Macrodeformation describes the actual shrinking of the defect since the edges are drawn together by the deforming force of the sponge. This leads to collapsing of the preformed holes in the mediastinum or thorax.
Microdeformation on the other hand occurs on a cellular level. Due to mechanical forces, the deformed cytoskeleton initiates intracellular signaling cascades leading to the release of growth factors, upregulation of granulation tissue formation and stretching of the cytoskeleton. Thereby, cell proliferation and migration are promoted (16,21).
Improvement of perfusion
Essential for healing of the defect is sufficient oxygen and nutrient supply afforded by increased blood flow. By inducing low perfusion and hypoxia in the defects’ edges, angiogenesis promoting mediators such as hypoxia-inducible factor 1α and vascular endothelial growth factor are locally released by affected cells (22,23). Thus, vessel density increases significantly leading to better perfusion, blood supply and ultimately optimum healing conditions (24).
Procedure
In order to place the sponge at its destination, a nasogastric tube (NGT) is used. The NGT consist of a silicon tube and an open-pore polyurethane foam attached to the tubes head.
Before inserting the NGT, exact evaluation of the defect regarding its dimension must be made endoscopically. The inserted endoscope is at first used for exact determination of the defects’ height and secondly used to estimate its size. The sponge size should be individually chosen or cut and prepared onto the tip of the silicon tube. Important to consider is the limitation of the sponges’ size due to the diameter of the esophagus. Oversized sponges can hardly be placed under vision and hinder exact positioning. Alternatively, specialized systems like Eso-SPONGE can be used equally (B. Braun Melsungen AG, Melsungen, Germany).
After choosing the right size, a second tube of larger width is placed as splinting catheter or “over tube” ending directly at the defect. Then, in order to prevent damage to the upper esophageal sphincter, the NGT is pushed through the over tube to its final destination.
In case of a defect without or small extramural cavity, it can be placed directly into the lumen of the esophagus (intraluminal). In this case, frequently a long, cylindrical sponge should be used. If an extraluminal cavity of the leakage exist, the preferred placement should be into the cavity (intracavitary) (25). In order to avoid folding of the sponge making it less effective, a shorter, thicker sponge is preferred. If the size of the sponge does not fill the cavity sufficiently, additional sponges can be applied. The lumen of the cavity will collapse, and exudative fluid removed when starting suction.
Afterwards, the pushing instrument and the large tube should be removed.
Final placement can be controlled and corrected under endoscopic visualization with an endoscopic forceps or grasper.
Then, the sponge tube is put from oral to nasal position and continuous suction is applied. Therefore, the NGT is connected to an electronic vacuum pump at a defined vacuum and continuous moderate intensity. The optimal intensity of the suction (mmHg) is under discussion and no evidenced based recommendation is available. A routinely applied vacuum of 125 mmHg is reported, however, vacuum intensity can be varied depending on individual preference. If patients do not tolerate suction well, settings can be changed to intermittent suction (5 min on, 2 min off) at the same pressure.
An alternative way of placing the EVAC, especially used for children, is putting it directly with a forceps to its final destination. The sponge should be lubricated and provided with a prolene stitch at its top to enable grasping and proper positioning. Additionally, the sponge can be placed retrograde through an existing gastrostomy (26). The sponge is put through the gastrostomy and pulled out of the mouth with a forceps. Then, it can be placed into position under fluoroscopic guidance.
If desired, an additional feeding tube can be placed before. Although there is limited data to oral fluid intake during EVAC therapy, especially patients with intraluminal sponges should avoid it strictly.
Just like common VAC therapy, the sponge of EVAC therapy should ideally remain 3–5 d; however, no more than 7 days until replacement. The sponge tends stick to the surrounding tissue making removal harder after some days. Every exchanging procedure the defect must be reevaluated, and the necessity of a new cycle decided. EVAC therapy is completed when the leakage closed completely, or the leakage cavity is well sealed off. Most studies report treatment durations around 15 days (27). However, depending on individual preconditions, treatment duration can vary and should be decided individually (Figures 1,2).
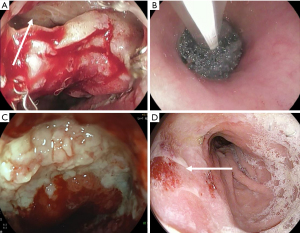
Stent-Over-Sponge (SOS)
A further advancement of the EVAC therapy is the SOS concept, first reported in Germany in 2018 (28). Thereby, after placing the NGT with the sponge onto the defect, a partially covered self-expandable metal stent is positioned over the sponge. The idea of this adjustment is potential improved vacuum force from the stents’ pressure and maintenance of esophageal passage. In the first small series of 11 patients, this approach has proven to be safe and feasible. It was successfully applied as second-line therapy after failed previous EVAC therapy. However, for clear clinical benefit of this new approach, probably as fist-line therapy, enough evidence is lacking.
Efficiency and comparison to SEMS
Successful EVAC therapy used for anastomotic leakages after esophagectomy has been reported in several case series and systematic reviews (29). First introduced with two successful case reports in 2008 and 2009 by Wedemeyer et al. and Loske et al. (14,30), first case series followed soon. In 2016, Laukoetter et al. treated 39 patients for anastomotic leakage with EVAC and reached successful healing rates of 92.3% (31). Min et al. presented 19 of 20 successfully treated patients with EVAC in 2019 (healing rate of 95%). In this study two factors, neoadjuvant therapy and the size of the defect, led to longer treatment duration (27).
Many cohort studies compared EVAC therapy to SEMS therapy indicating equal or better efficiency. In a retrospective study in 2018, Berlth et al. analyzed 101 patients either treated with SEMS or EVAC (32). They found no superior outcome for either therapeutic option. On the other hand, another retrospective analysis of 45 patients treated either with EVAC or SEMS by Mennigen et al. describes statistically significant better healing rates for the initial vacuum therapy (endoscopic vacuum 93.3%, stent 63.3%; P=0.038). Thirty of the patients received stent therapy, 15 underwent EVAC therapy. Seven patients in the SEMS group were switched to EVAC and four to surgery because of treatment failure. Comparing healing rates of the final therapy, EVAC still reached better outcome (EVAC, 86.4%; SEMS, 60.9%; P=0.091) (15). In a cohort study with 71 patients from Brangewitz in 2013, the overall closure rate was significantly higher in the EVAC group (84.4%) compared with the SEMS/SEPS group (53.8%) (33). Finally, a retrospective study of Schniewind et al. in 2013 evaluated different treatment regiments for anastomotic leakage after esophagectomy. Patients treated with endoluminal vacuum therapy had significant lower mortality rates than patients treated with surgery or stent implantation (34). The first meta-analysis conducted, confirmed these findings (35). Still, randomized controlled trials proving superiority of this concept are missing in current literature.
Possible advantages of EVAC
Apart from the proven efficiency, EVAC therapy offers additional advantages. Due to regular sponge exchanges and visualization of the defect, a potential healing problem or deterioration can be managed at an early stage. This on the other hand leads to more interventions and resource consumption compared to stent therapy. As additional important advantage, the EVAC system provides adequate drainage of the wound and control of the infectious site, avoiding sepsis. Therefore, placement of additional drainages and possible complications caused by these interventions (e.g., by interventional radiology) can be obviated.
The sponge can be individually prepared and adapted to various possible defects of the intrathoracic esophagus. Even advanced defects can be treated endoscopically and drainage along the whole esophagus can be provided. In contrast to SEMS therapy, adaption is tension-free at any time, whereas the stiff forces of a stent might hinder wound margins to approximate and heal (33). This stiffness can also cause circular esophageal ulceration leading to formation of scar tissue and anastomotic structure.
As disadvantage of the EVAC therapy the postponed oral feeding has to be mentioned. If endoluminal EVAC is applied, enteral feeding can only be applied by another nasojejunal feeding catheter. Successful placement of SEMS allows oral feeding with fluid and soft foods.
Safety
Taking the published reviews into account, EVAC therapy generally is a safe procedure. Possible minor adverse events include sponge dislocation and minor bleeding after sponge exchange. Sponge dislocation often occurs in a late treatment phase when the cavity has already healed well, and the sponge is placed intraluminally. Coughing and swallowing can then easily cause dislocation. Minor bleedings are mostly self-limiting and can be mitigated by increasing exchange frequency (36).
Regarding post-interventional anastomotic strictures, Min et al. report a 35.0% anastomotic stenosis rate after successful EVAC treatment. These strictures clinically present with dysphagia and are successfully treated by repeated endoscopic balloon dilatation. Other studies reported lower stricture rates following EVAC therapy: Brangewitz et al. reported 9.4% (3 of 32 cases), Laukoetter et al. reported 7.7% (4 of 52 cases), and Schorsch reported 4.2% (1 of 24 cases) rates. However, those studies included other cases of esophageal perforation apart from anastomotic leakages. Taken the underlying cohort as possible bias into consideration, the anastomotic stricture rates after treatment of anastomotic leakage with EVAC might be underestimated.
The most threatening complications of EVAC described in literature are major bleeding due to direct neighborhood of big vessels. In a review of Rausa et al. with 163 included patients, two patients in the endoscopic vacuum treatment goup (2.8%) developed fistula between cavity and blood system leading to major bleeding (35). In their study, Pournaras et al report one case of bleeding due to direct communication of an aortic with the cavity. Placing of an aortic stent could stop the bleeding (37). In the cohort study of Laukoetter et al. two patients died from massive hemorrhage, again caused by close contact to cardiovascular structure (31). They suggest exact pre-interventional evaluation of anatomic sites by CT-imaging in order to avoid such events. However, an increased provocation of fistula between GI system and airway has not been described (32). In the end, these cases illustrate the need for structured, evidence-based guidelines allowing safe and successful treatment with EVAC.
Comparing EVAC to SEMS, most studies report lower complication rates in the EVAC group (38). Stent placement SEMS often requires re-endoscopy because of stent migration and necessity of replacement. Additionally, since the stent remains longer in situ during stent therapy—treatment duration difference of 9 days was found in a meta-analysis (35)—its removal can be extra difficult due to overgrowing tissue, which can lead more postinterventional stenosis or more severe complication like broncho-esophageal fistula (38). Although the same complication might happen during EVAC therapy, a regular sponge exchange after 5 days can prevent these complications.
Cost and duration
In times of increasing health care cost, economic proficiency should also be considered. In terms of treatment duration, most studies found faster leakage closure with EVAC therapy. Still, in order to evaluate proficiency, a comparison to the alternative approach, SEMS implementation, must be conducted. Rausa et al. found a pooled mean treatment duration difference of −9 days (95% CI: 16.6–1.4; P=0.021) in favor of EVAC therapy (35).
Another aspect should be the time needed by an endoscopist to reach maximum proficiency as flat learning curves indicate less economic procedures. Ward et al. analyzed medium time needed to reach a plateau of average procedure time performing EVAC procedure. An experienced endoscopist obtained this plateau of roughly 43 min after 10 procedures with lower costs when executed in the GI lab (39). This number does not seem high considering performance of approximately four procedures for every patient (38). Although the procedure reaches proficiency fast, it inevitable includes interventions (sponge exchanges) on a regular basis resulting in overall higher therapeutic costs.
Baltin et al. analyzed 39 patients treated in Germany between January 2012 and December 2016 with either SEMS or EVAC therapy regarding the average economic burden of both interventions (40). Treatment for patients in the EVAC group caused almost double costs. From an economic point of view, although healing faster with a steep learning curve, EVAC has an inferior standing. However, higher failure rate of stent therapy might lead to costlier second line therapies such as surgery, making EVAC the better choice from the start.
Conclusions
Anastomotic leakage is a common but most burdensome complication after esophagectomy. Mortality can be mitigated by improving complication management. Although SEMS is an easily reproducible method with acceptable success rates, in recent literature EVAC therapy presents as a convincing, safe, and even more efficient alternative. Since for today official guidelines regarding adequate pressure, optimum exchange cycle and treatment set up are missing, many centers have established their own treatment algorithm. Prospective randomized trials are required to reach a consensus about ideal therapeutic strategy. Nevertheless, although being not as cost efficient as SEMS therapy, EVAC therapy should be valued as an important part of complication management for anastomotic leakages following esophagectomy.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Alejandro Nieponice) for the series “Anastomotic Techniques for Minimally Invasive Esophagectomy and Endoscopic Handling of Its Complications” published in Annals of Esophagus. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-16/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-16/coif). The series “Anastomotic Techniques for Minimally Invasive Esophagectomy and Endoscopic Handling of Its Complications” was commissioned by the editorial office without any funding or sponsorship. PG serves as an unpaid editorial board member of Annals of Esophagus from September 2020 to August 2022. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Triantafyllou T, Wijnhoven BPL. Current status of esophageal cancer treatment. Chin J Cancer Res 2020;32:271-86. [Crossref] [PubMed]
- Mann C, Berlth F, Hadzijusufovic E, et al. Minimally invasive esophagectomy: clinical evidence and surgical techniques. Langenbecks Arch Surg 2020;405:1061-7. [Crossref] [PubMed]
- van der Sluis PC, Tagkalos E, Hadzijusufovic E, et al. Robot-Assisted Minimally Invasive Esophagectomy with Intrathoracic Anastomosis (Ivor Lewis): Promising Results in 100 Consecutive Patients (the European Experience). J Gastrointest Surg 2021;25:1-8. [Crossref] [PubMed]
- Grimminger PP, Goense L, Gockel I, et al. Diagnosis, assessment, and management of surgical complications following esophagectomy. Ann N Y Acad Sci 2018;1434:254-73. [Crossref] [PubMed]
- Urschel JD. Esophagogastrostomy anastomotic leaks complicating esophagectomy: a review. Am J Surg 1995;169:634-40. [Crossref] [PubMed]
- Sarela AI, Tolan DJ, Harris K, et al. Anastomotic leakage after esophagectomy for cancer: a mortality-free experience. J Am Coll Surg 2008;206:516-23. [Crossref] [PubMed]
- Walther B, Johansson J, Johnsson F, et al. Cervical or thoracic anastomosis after esophageal resection and gastric tube reconstruction: a prospective randomized trial comparing sutured neck anastomosis with stapled intrathoracic anastomosis. Ann Surg 2003;238:803-12; discussion 812-4. [Crossref] [PubMed]
- Verstegen MHP, Bouwense SAW, van Workum F, et al. Management of intrathoracic and cervical anastomotic leakage after esophagectomy for esophageal cancer: a systematic review. World J Emerg Surg 2019;14:17. [Crossref] [PubMed]
- Rizk NP, Bach PB, Schrag D, et al. The impact of complications on outcomes after resection for esophageal and gastroesophageal junction carcinoma. J Am Coll Surg 2004;198:42-50. [Crossref] [PubMed]
- Van Daele E, Van de Putte D, Ceelen W, et al. Risk factors and consequences of anastomotic leakage after Ivor Lewis oesophagectomy†. Interact Cardiovasc Thorac Surg 2016;22:32-7. [Crossref] [PubMed]
- Goense L, van Rossum PSN, Weijs TJ, et al. Aortic Calcification Increases the Risk of Anastomotic Leakage After Ivor-Lewis Esophagectomy. Ann Thorac Surg 2016;102:247-52. [Crossref] [PubMed]
- Nirula R. Esophageal perforation. Surg Clin North Am 2014;94:35-41. [Crossref] [PubMed]
- Rodrigues-Pinto E, Pereira P, Ribeiro A, et al. Self-expanding metal stents in postoperative esophageal leaks. Rev Esp Enferm Dig 2016;108:133-7. [Crossref] [PubMed]
- Wedemeyer J, Schneider A, Manns MP, et al. Endoscopic vacuum-assisted closure of upper intestinal anastomotic leaks. Gastrointest Endosc 2008;67:708-11. [Crossref] [PubMed]
- Mennigen R, Harting C, Lindner K, et al. Comparison of Endoscopic Vacuum Therapy Versus Stent for Anastomotic Leak After Esophagectomy. J Gastrointest Surg 2015;19:1229-35. [Crossref] [PubMed]
- Saxena V, Hwang CW, Huang S, et al. Vacuum-assisted closure: microdeformations of wounds and cell proliferation. Plast Reconstr Surg 2004;114:1086-96; discussion 1097-8. [Crossref] [PubMed]
- Nagell CF, Holte K. Treatment of anastomotic leakage after rectal resection with transrectal vacuum-assisted drainage (VAC). A method for rapid control of pelvic sepsis and healing. Int J Colorectal Dis 2006;21:657-60. [Crossref] [PubMed]
- Lalezari S, Lee CJ, Borovikova AA, et al. Deconstructing negative pressure wound therapy. Int Wound J 2017;14:649-57. [Crossref] [PubMed]
- Orgill DP, Bayer LR. Negative pressure wound therapy: past, present and future. Int Wound J 2013;10:15-9. [Crossref] [PubMed]
- Mouës CM, Vos MC, van den Bemd GJ, et al. Bacterial load in relation to vacuum-assisted closure wound therapy: a prospective randomized trial. Wound Repair Regen 2004;12:11-7. [Crossref] [PubMed]
- Orgill DP, Bayer LR. Update on negative-pressure wound therapy. Plast Reconstr Surg 2011;127:105S-115S. [Crossref] [PubMed]
- Erba P, Ogawa R, Ackermann M, et al. Angiogenesis in wounds treated by microdeformational wound therapy. Ann Surg 2011;253:402-9. [Crossref] [PubMed]
- Greene AK, Puder M, Roy R, et al. Microdeformational wound therapy: effects on angiogenesis and matrix metalloproteinases in chronic wounds of 3 debilitated patients. Ann Plast Surg 2006;56:418-22. [Crossref] [PubMed]
- Malsiner CC, Schmitz M, Horch RE, et al. Vessel transformation in chronic wounds under topical negative pressure therapy: an immunohistochemical analysis. Int Wound J 2015;12:501-9. [Crossref] [PubMed]
- Loske G, Schorsch T, Müller C. Intraluminal and intracavitary vacuum therapy for esophageal leakage: a new endoscopic minimally invasive approach. Endoscopy 2011;43:540-4. [Crossref] [PubMed]
- Manfredi MA, Clark SJ, Staffa SJ, et al. Endoscopic Esophageal Vacuum Therapy: A Novel Therapy for Esophageal Perforations in Pediatric Patients. J Pediatr Gastroenterol Nutr 2018;67:706-12. [Crossref] [PubMed]
- Min YW, Kim T, Lee H, et al. Endoscopic vacuum therapy for postoperative esophageal leak. BMC Surg 2019;19:37. [Crossref] [PubMed]
- Valli PV, Mertens JC, Kröger A, et al. Stent-over-sponge (SOS): a novel technique complementing endosponge therapy for foregut leaks and perforations. Endoscopy 2018;50:148-53. [Crossref] [PubMed]
- Jeon JH, Jang HJ, Han JE, et al. Endoscopic Vacuum Therapy in the Management of Postoperative Leakage After Esophagectomy. World J Surg 2020;44:179-85. [Crossref] [PubMed]
- Loske G, Müller C. Vacuum therapy of an esophageal anastomotic leakage--a case report. Zentralbl Chir 2009;134:267-70. [Crossref] [PubMed]
- Laukoetter MG, Mennigen R, Neumann PA, et al. Successful closure of defects in the upper gastrointestinal tract by endoscopic vacuum therapy (EVT): a prospective cohort study. Surg Endosc 2017;31:2687-96. [Crossref] [PubMed]
- Berlth F, Bludau M, Plum PS, et al. Self-Expanding Metal Stents Versus Endoscopic Vacuum Therapy in Anastomotic Leak Treatment After Oncologic Gastroesophageal Surgery. J Gastrointest Surg 2019;23:67-75. [Crossref] [PubMed]
- Brangewitz M, Voigtländer T, Helfritz FA, et al. Endoscopic closure of esophageal intrathoracic leaks: stent versus endoscopic vacuum-assisted closure, a retrospective analysis. Endoscopy 2013;45:433-8. [Crossref] [PubMed]
- Schniewind B, Schafmayer C, Voehrs G, et al. Endoscopic endoluminal vacuum therapy is superior to other regimens in managing anastomotic leakage after esophagectomy: a comparative retrospective study. Surg Endosc 2013;27:3883-90. [Crossref] [PubMed]
- Rausa E, Asti E, Aiolfi A, et al. Comparison of endoscopic vacuum therapy versus endoscopic stenting for esophageal leaks: systematic review and meta-analysis. Dis Esophagus 2018; [Crossref] [PubMed]
- Loske G, Müller CT. Tips and tricks for endoscopic negative pressure therapy. Chirurg 2019;90:7-14. [Crossref] [PubMed]
- Pournaras DJ, Hardwick RH, Safranek PM, et al. Endoluminal Vacuum Therapy (E-Vac): A Treatment Option in Oesophagogastric Surgery. World J Surg 2018;42:2507-11. [Crossref] [PubMed]
- Hwang JJ, Jeong YS, Park YS, et al. Comparison of Endoscopic Vacuum Therapy and Endoscopic Stent Implantation with Self-Expandable Metal Stent in Treating Postsurgical Gastroesophageal Leakage. Medicine (Baltimore) 2016;95:e3416. [Crossref] [PubMed]
- Ward MA, Hassan T, Burdick JS, et al. Endoscopic vacuum assisted wound closure (EVAC) device to treat esophageal and gastric leaks: assessing time to proficiency and cost. Surg Endosc 2019;33:3970-5. [Crossref] [PubMed]
- Baltin C, Kron F, Urbanski A, et al. The economic burden of endoscopic treatment for anastomotic leaks following oncological Ivor Lewis esophagectomy. PLoS One 2019;14:e0221406. [Crossref] [PubMed]
Cite this article as: Mann C, Berlth F, Tagkalos E, Hadzijusufovic E, Lang H, Grimminger P. Endoscopic management of complications—endovacuum for management of anastomotic leakages: a narrative review. Ann Esophagus 2022;5:15.


