
Management of esophageal perforations and postoperative leaks
Esophageal perforations
Since its first description in 1723 by Boerhaave, esophageal perforation has been remaining a life-threatening and challenging event, with a controversial management and still high mortality.
Perforation results from a full-thickness tear in the esophageal wall due to a trauma on the esophageal lumen or to a sudden increase of endoluminal pressure combined with relatively negative intrapleural pressure, like in spontaneous perforations.
Iatrogenic injury to the esophagus during endoscopy is the most frequent cause, accounting for 59% of all cases (1), followed by spontaneous perforations (15%), foreign body or caustic ingestion (12%), trauma (9%), operative injuries (2%), tumors (1%) and other causes (2%).
If not diagnosed and correctly managed in the first 24 h, mortality rate after esophageal perforation can range from 4% to 80% (2).
The onset of symptoms varies depending on the location of perforation (cervical, intrathoracic or intraabdominal), the degree of tissue destruction and extension of contamination of surrounding organs, the cause, the timing of diagnosis and the presence of underlying esophageal disorders (1,3).
The most frequent symptom is chest pain (in 70–90% of patients) usually irradiated between the shoulder blades and the neck, which generally indicates the site of perforation. Other symptoms include: dyspnea, dysphagia, sialorrhea, odynophagia (manly in the neck perforations), subcutaneous emphysema (4,5). Later symptoms and signs are: fever, tachycardia, pleural effusion, cough, hypotension and respiratory compromise till the septic shock (6).
In Boerhaave’s syndrome, a classical described presentation is the Mackler’s triad, that includes chest pain, vomiting, and subcutaneous emphysema, nevertheless it is only present in 14% of people (7).
The overall mortality depends on the etiology, location of the injury and delay in diagnosis and correct treatment. In a comprehensive review by Brinster et al. (1) of 9 recent series on 431 patients, mortality is 36% (0–72%) after spontaneous esophageal perforation, 19% (7–33%) after iatrogenic causes and 7% (0–33%) in case of trauma. The higher mortality is also recorded after thoracic perforation (27%, 0–44%), rather than abdominal (21%, 0–43%) or cervical (6%, 0–16%) (1). The difference is explained by a major containment of contamination after esophageal perforation in cervical district by fascial plane of the neck compared to mediastinal or abdominal perforation, where a septic status is more frequent. If treatment is delayed over 24 h the mortality is 27% (0–46%) compared to 14% (0–28%) when the perforation is treated within 24 h, as reported by Brinster (1) reviewing 390 cases from 11 published series.
Indeed, outcomes seem to be affected by ability to diagnose the site of perforation and start the most appropriate treatment rapidly within the first 24 h.
Instrumental confirmation of clinical diagnosis is done by X-ray with water-soluble contrast medium (Gastrografin®), the most used for its accuracy [only 10% of false negatives, (1)] in detecting the location and extension of perforation. Computed tomography (CT) scan is useful in case of difficult perforations to be located of when patient’s conditions prevent execution of contrast esophagography. The main CT findings suggestive for perforation are: pneumomediastinum, esophageal thickening, mediastinal air-fluid collection or abscess, pleural effusion or pneumothorax (8,9). For enhancing accuracy of CT, when feasible, gastrografin can be taken per os. Flexible esophagoscopy is associated with a 100% sensitivity and 83% specificity for emergent diagnosis of traumatic perforations (1). Its role in non-penetrating perforation is still debated (1) because the use of air insufflation may exacerbate small mucosal tears and facilitate perforation and surrounding contamination. In case of pleural effusion, the chemical examination of fluid can show the presence of salivary amylase, food or a pH of less than 6.0, all diagnostic for esophageal perforation.
Treatment
The main parameters to be evaluated for the correct management of esophageal perforations are: the location, the cause, the extension, the interval between perforation and treatment, the age of the patient, concomitant comorbidities and general health status (1).
According to Kuppusamy et al. (2) and Sepesi et al. (6), referral to a tertiary care center is crucial for correct treatment of esophageal perforations in the first critical 24 h. Furthermore, the surgical leadership of the multidisciplinary team is fundamental to use a diversified approach, including operative and non-operative techniques, in order to reduce mortality and improve outcomes. Non-operative management includes nothing per os, parental or enteral nutrition, and broad-spectrum antibiotic therapy. This approach was first successfully described by Mengold and Klassen (10) in 1965, followed by the report by Larrieu and Kieffer (11) for spontaneous perforations, in 1975.
Altorjay and colleagues (12) and others (4,13) suggested the following criteria for adopting a conservative management of perforations: early diagnosis of intramural perforation, transmural perforation within neck/mediastinum if diagnosis delayed, perforation not in neoplastic tissue/abdomen/proximal to obstruction, no evidence of sepsis and the availability of thoracic surgeons and contrast imaging. Recently, the Pittsburgh group (14) proposed a 10-variable severity score for esophageal perforation, evaluating: age, heart rate, fever, pleural effusion, leukocytes, non-concomitant leak, respiratory situation, timing to diagnosis, hypotension and presence of cancer. Patients with minimal score (no mediastinal contamination and no respiratory compromise) can be managed with non-operative measures, having better outcomes than when managed surgically. Historically, it was believed that the only variable affecting outcomes was the ability to detect and treat the perforation within the first 24 h. As showed by Kuppusamy et al. (2) on a series of 81 patients, although an early detection is important, the crucial point in esophageal perforation outcome is the initial management by a multidisciplinary team led by experienced surgeons in tertiary centers. In this way, a wide number of hybrid (surgical and interventional), conservative or surgical procedures can be used in order to reduce the necessity of esophageal exclusion procedures and improve outcomes in terms of morbidity, mortality and in-hospital stay. Furthermore, this approach led to an increasing of non-operative therapy from 0 to 75%, with a reduction of complications (50% to 33%) and 4% of mortality (2). They also proposed a therapeutic algorithm for management of acute esophageal perforations; however, it is not possible and not suggested to have a standardized therapeutic pathway because this complex and insidious pathology requires an individualized approach for each patient (15). Nevertheless, in general, in patient in stable clinical condition, with early detected and contained perforation (like in case of iatrogenic one), without any sign of sepsis or organ failure, a conservative treatment may be adopted.
The recent introduction and development of removable plastic/covered metal esophageal stent technology represents an important weapon available for non-surgical treatments. However, for the use of this technology absent or minimal contamination of mediastinum is mandatory. In case of significant contamination, the use of stents may be help to avoid an esophageal resection/exclusion in patients not fit for reconstruction only if associated to a surgical debridement and drainage of mediastinum or pleural cavity. A recent review of Sepesi et al. (6) collected several series on successful use of stents, reporting a coverage rate of the leak from 78% to 92%, a stent migration rate of 3–21%, and a mortality rate of 6–15.6%.
A novel strategy for endoscopic treatment of esophageal perforations and fistula is endoscopic vacuum therapy (EVT) that seems to promote healing of esophageal defects and reduce septic status (16) in patients that can’t undergo surgical treatments.
Failure of conservative or hybrid treatments, large perforations with massive contamination of mediastinum and septic status or the presence of underlying pathology (like stricture, end-stage achalasia, cancer) requires surgical management, when feasible.
The first successful surgical repairs of esophagus were described by Barrett (17), Olsen and Clagett (18) in 1947. Since then, the improvement of surgical techniques and anesthetic management allowed the treatment of complex situations. Surgical approach depends on the localization of perforation.
Cervical perforations, that generally are not lethal due to the containment of surrounding neck structures (6), can be managed through left cervical incision, and often require only drainage of purulent material and possible esophageal mucosal suture.
Injuries of upper and medium esophagus are usually approached by right thoracotomy (V intercostal space), while distal esophageal perforations are better managed through left thoracotomy (VI intercostal space). In the last years, the role of thoracoscopy for managing esophageal perforation has been improving, allowing a faster recovery of the patients (1,19). Surgical treatment consists of debridement of necrotic tissue and toilette of purulent material (Figure 1) to expose the perforation. In early treatment, the attempt of suture can be successful and requires a proximal and distal myotomy to expose the mucosal rupture, that must be repaired by interrupted absorbable suture. Then the esophageal muscle must be closed with another interrupted suture, better if buttressed with vascularized flap of intercostal muscle, diaphragmatic pedicle, pleural or omental graft (1,6). When a repair is not feasible, like in delayed context or in the setting of extensive necrotic tissue, a hybrid treatment can be attempted: after surgical toilette and drainage of mediastinum and pleural space, a stent or a nasoesophageal tube can be placed to avoid directly an esophageal resection or diversion. Several exclusion and diversion techniques are described (5). Conventionally, a proximal and distal diversion is performed to exclude the perforated tract and an end or side cervical esophagostomy is made. This technique requires a second stage of reconstruction. To eliminate the need of a second operation, a proximal esophageal ligation can be done with creation of a cervical esophageal mucosa fistula (side esophagostomy) (1).
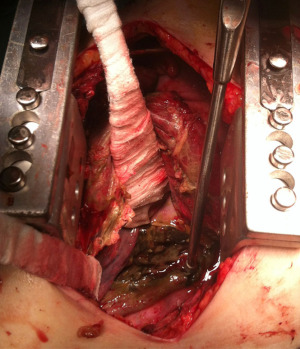
In case of concomitant underlying pathology, like cancer or esophageal strictures or end-stage achalasia, an esophageal resection, a neck esophagostomy and a distal feeding tube (like jejunostomy) are suggested, with delayed reconstruction of the digestive tract after resolution of mediastinal contamination and septic status (6).
Surgical esophageal reconstruction can be done by trans-hiatal approach, with one stage esophagectomy and reconstruction but only in case of early diagnosis and minimal mediastinal or pleural contamination (1). Transthoracic reconstruction gives the possibility of pleural decortication and mediastinal toilette. The decision of reconstruction timing is clinical and can be performed by gastric tubulization and pull-up or colic transposition. In case of thickened and difficult mediastinum like after esophagectomy for caustic injection (20), or after necrosis of gastric conduit (21), a retrosternal route may be preferred.
The anastomosis between esophagus and the transposed digestive conduit can be performed intrathoracic [according to Ivor-Lewis technique (21)], in case only the distal part of the esophagus is resected, or cervical (according to Mc Keown technique). Orringer and Stirling (22,23) proposed a cervical semi-mechanical end-to side esophagogastric anastomosis, whose posterior wall is made by a mechanical suture and the anterior wall by interrupted absorbable suture, to reduce risk of stricture. In case of anastomotic leakage, it can be managed only by opening cervical incision.
Post-operative leaks
Among esophageal “injuries”, a separate discussion must be reserved for post-operative esophageal leaks, having a different origin but a quite similar clinical presentation and treatment to esophageal perforations. The reported incidence is 6.6–17.2% (24) in case of cervical esophagogastric anastomosis and 2–15.9% in intrathoracic anastomosis [9.8% after transthoracic esophagectomy and 12% after trans-hiatal one (25)]. Mortality ranges from 17 to 35% in patients with leak after esophagectomy and there is also evidence of worst long-term oncological prognosis (26).
Several risk factors are involved in the development of anastomotic leakage, like: surgical technique and expertise, location of the anastomosis, preoperative radiotherapy, malnutrition, diabetes and corticosteroid use (24). Mechanical anastomoses with linear stapler seem to be associated to less risk of fistula and stricture compared to manual ones (27,28); cervical localization is also associated to a higher risk of leakage (29,30) but to lower mortality and can be managed by opening the cervical incision (Figure 2A,2B).
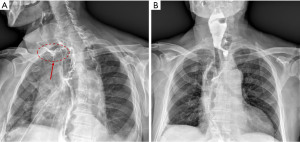
In 2015, the Consensual Complications Group for Esophagectomy (ECCG) proposed a classification for esophageal anastomotic leaks, defined as total parietal defects, involving esophagus, anastomosis and gastric tube.
Type 1 is treated medically and does not require any modification in therapy; type 2 that requires interventional/radiological treatment but not surgery and type 3 that requires surgery (25).
Often anastomotic fistula is due to gastric tube necrosis. Type 1 gastric necrosis is focal, it is identified endoscopically and needed only medical therapy; type 2 is a focal necrosis that requires a surgical operation but not an esophageal diversion, like in type 3 (extensive gastric tube necrosis).
As for esophageal perforations, prompt diagnosis of postoperative fistula is crucial and improves outcomes. Early clinical signs are: pyrexia, tachycardia, increased neutrophil count (in the first phase), increased C-reactive protein, cardiac arrhythmias, subcutaneous emphysema (in case of cervical anastomosis), pneumomediastinum or pneumothorax (for thoracic anastomosis) or air-leaking from surgical drains. Late signs are biliary, purulent or necrotic fluid from drainages, hypotension and all signs of septic shock.
Treatment
As for the esophageal perforations, after radiological and endoscopic confirmation of esophageal leak, the first steps are nihil per os, antibiotic therapy and local drainage.
Small anastomotic leaks can be treated conservatively, with fasting and nasoesophageal tube in aspiration. In case of mediastinal or pleural contamination, surgical toilette is mandatory.
When feasible endoscopic treatments can be effective and minimally invasive, like placement of transluminal vacuum therapy (useful in case of mediastinitis), or covered stents (2), associated to a 72% of efficiency. Endoscopic suturing device and clips are also used in the last years with success (6). Large anastomotic leaks associated to septic status or necrosis of gastric conduit require an urgent surgical esophageal diversion with cervical esophagostomy. In case of removal of necrotic gastric tube, esophageal reconstruction must be planned after improvement of patient clinical condition.
Our experience
In our personal thirty-year experience as tertiary center, the management of esophageal perforation was always based on the principles reported in the present review, employing conservative/endoscopic treatments when feasible or surgical debridement and esophageal diversion when required by patient clinical conditions. In all cases the prompt evaluation of the patient by an experienced multidisciplinary team allowed a customized treatment, with a mortality rate of 7% and a mean hospitalization of 35 days (9–150 days).
Recently, our group proposed (31) and has been still working on an innovative, promising and easily reproducible treatment of esophageal perforations by endoscopic injection around fistula borders of the emulsified adipose tissue stromal vascular fraction (tSVFem) obtained by mechanical manipulation of autologous fat tissue. The method is based on the regenerative-tissue capacity and anti-inflammatory power of adipose-derived stem cells contained in tSVFem and it has been showing interesting results in healing acute and chronic esophageal perforations and fistulas in all cases unfit for surgery or common endoscopic treatments.
Conclusions
In conclusion, esophageal perforations and leaks are challenging conditions and diagnostic timing is crucial. Indeed, early diagnosis and correct treatment can reduce mortality of 50%. Recent evidence shows how better outcomes are achieved when the management is multidisciplinary, led by an expert team, and individualized treatments are adopted, involving all available modalities (medical, endoscopic and surgical).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Esophagus for the series “Management of Esophageal Perforations and Injuries and Other Benign Diseases”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form, (available at https://aoe.amegroups.com/article/view/10.21037/aoe-2020-23/coif). The series “Management of Esophageal Perforations and Injuries and Other Benign Diseases” was commissioned by the editorial office without any funding or sponsorship. VP served as an unpaid Guest Editor of the series. DN served as an unpaid Guest Editor of the series and serves as an unpaid editorial board member of Annals of Esophagus from October 2019 to September 2021. The authors have no other conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Brinster CJ, Singhal S, Lee L, et al. Evolving options in the management of esophageal perforation. Ann Thorac Surg 2004;77:1475-83. [Crossref] [PubMed]
- Kuppusamy MK, Hubka M, Felisky CD, et al. Evolving management strategies in esophageal perforation: surgeons using nonoperative techniques to improve outcomes. J Am Coll Surg 2011;213:164-71; discussion 171-2. [Crossref] [PubMed]
- Bresadola V, Terrosu G, Favero A, et al. Treatment of perforation in the healthy esophagus: analysis of 12 cases. Langenbecks Arch Surg 2008;393:135-40. [Crossref] [PubMed]
- Bladergroen MR, Lowe JE, Postlethwait RW. Diagnosis and recommended management of esophageal perforation and rupture. Ann Thorac Surg 1986;42:235-9. [Crossref] [PubMed]
- Gouge TH, Depan HJ, Spencer FC. Experience with the Grillo pleural wrap procedure in 18 patients with perforation of the thoracic esophagus. Ann Surg 1989;209:612-7; discussion 617-9. [Crossref] [PubMed]
- Sepesi B, Raymond DP, Peters JH. Esophageal perforation: surgical, endoscopic and medical management strategies. Curr Opin Gastroenterol 2010;26:379-83. [Crossref] [PubMed]
- Woo KM, Schneider JI. High-risk chief complaints I: chest pain--the big three. Emerg Med Clin North Am 2009;27:685-712. x. [Crossref] [PubMed]
- Maher MM, Lucey BC, Boland G, et al. The role of interventional radiology in the treatment of mediastinal collections caused by esophageal anastomotic leaks. AJR Am J Roentgenol 2002;178:649-53. [Crossref] [PubMed]
- Backer CL, LoCicero J 3rd, Hartz RS, et al. Computed tomography in patients with esophageal perforation. Chest 1990;98:1078-80. [Crossref] [PubMed]
- Mengold LR, Klassen KP. Conservative management of esophageal perforation. Arch Surg 1965;91:232-40.
- Larrieu AJ, Kieffer R. Boerhaave syndrome: report of a case treated non-operatively. Ann Surg 1975;181:452-4. [Crossref] [PubMed]
- Altorjay A, Kiss J, Vörös A, et al. Nonoperative management of esophageal perforations. Is it justified? Ann Surg 1997;225:415-21. [Crossref] [PubMed]
- Shaffer HA Jr, Valenzuela G, Mittal RK. Esophageal perforation. A reassessment of the criteria for choosing medical or surgical therapy. Arch Intern Med 1992;152:757-61. [Crossref] [PubMed]
- Abbas G, Schuchert MJ, Pettiford BL, et al. Contemporaneous management of esophageal perforation. Surgery 2009;146:749-55; discussion 755-6. [Crossref] [PubMed]
- Vallböhmer D, Hölscher AH, Hölscher M, et al. Options in the management of esophageal perforation: analysis over a 12-year period. Dis Esophagus 2010;23:185-90. [Crossref] [PubMed]
- Mastoridis S, Chana P, Singh M, et al. Endoscopic vacuum therapy (EVT) in the management of oesophageal perforations and post-operative leaks. Minim Invasive Ther Allied Technol 2022;31:380-8. [Crossref] [PubMed]
- BARRETT NR. Report of a case of spontaneous perforation of the oesophagus successfully treated by operation. Br J Surg 1947;35:216-8. [Crossref] [PubMed]
- Olsen AM, Clagett OT. Spontaneous rupture of the esophagus; report of a case with immediate diagnosis and successful surgical repair. Postgrad Med 1947;2:417-21. [Crossref] [PubMed]
- Elliott JA, Buckley L, Albagir M, et al. Minimally invasive surgical management of spontaneous esophageal perforation (Boerhaave's syndrome). Surg Endosc 2019;33:3494-502. [Crossref] [PubMed]
- Chirica M, de Chaisemartin C, Munoz-Bongrand N, et al. Colonic interposition for esophageal replacement after caustic ingestion. J Chir (Paris) 2009;146:240-9. [Crossref] [PubMed]
- Abe T, Fukaya M, Nagino M. Retrosternal salvage reconstruction of esophageal discontinuity for a necrotic gastric tube after esophagectomy: A novel procedure. J Med Invest 2018;65:296-8. [Crossref] [PubMed]
- Bains MS. Ivor Lewis esophagectomy. Chest Surg Clin N Am 1995;5:515-26.
- Orringer MB, Stirling MC. Cervical esophagogastric anastomosis for benign disease. Functional results. J Thorac Cardiovasc Surg 1988;96:887-93.
- Famiglietti A, Lazar JF, Henderson H, et al. Management of anastomotic leaks after esophagectomy and gastric pull-up. J Thorac Dis 2020;12:1022-30. [Crossref] [PubMed]
- Birla R, Hoara P, Dinu D, et al. Postoperative Esophageal Leaks in Malignant Pathology - Optimal Management: A Systematic Review. Chirurgia (Bucur) 2019;114:429-36. [Crossref] [PubMed]
- Turrentine FE, Denlinger CE, Simpson VB, et al. Morbidity, mortality, cost, and survival estimates of gastrointestinal anastomotic leaks. J Am Coll Surg 2015;220:195-206. [Crossref] [PubMed]
- Price TN, Nichols FC, Harmsen WS, et al. A comprehensive review of anastomotic technique in 432 esophagectomies. Ann Thorac Surg 2013;95:1154-60; discussion 1160-1. [Crossref] [PubMed]
- Markar SR, Arya S, Karthikesalingam A, et al. Technical factors that affect anastomotic integrity following esophagectomy: systematic review and meta-analysis. Ann Surg Oncol 2013;20:4274-81. [Crossref] [PubMed]
- Kassis ES, Kosinski AS, Ross P Jr, et al. Predictors of anastomotic leak after esophagectomy: an analysis of the society of thoracic surgeons general thoracic database. Ann Thorac Surg 2013;96:1919-26. [Crossref] [PubMed]
- Biere SS, Maas KW, Cuesta MA, et al. Cervical or thoracic anastomosis after esophagectomy for cancer: a systematic review and meta-analysis. Dig Surg 2011;28:29-35. [Crossref] [PubMed]
- Porziella V, Nachira D, Boškoski I, et al. Emulsified stromal vascular fraction tissue grafting: a new frontier in the treatment of esophageal fistulas. Gastrointest Endosc 2020;92:1262-3. [Crossref] [PubMed]
Cite this article as: Nachira D, Sassorossi C, Petracca-Ciavarella L, Zanfrini E, Tabacco D, Pogliani L, Meacci E, Congedo MT, Vita ML, Chiappetta M, Margaritora S, Porziella V. Management of esophageal perforations and postoperative leaks. Ann Esophagus 2023;6:10.


