
Enhanced recovery after surgery pathway in esophagectomy in a high volume center: clinical keys to early leak diagnosis after esophagectomy
Introduction
Esophageal cancer is an aggressive cancer which is associated with a poor survival. The standard of care for patients with locally advanced esophageal cancer is neo-adjuvant chemo(radio)therapy followed by radical esophagectomy with lymphadenectomy (1). Anastomotic leakage remains a frequently encountered complication with reported frequency ranging from 11.4% up to 30% (2,3). Because of the potentially harmful effect of extravasation of gastro-intestinal contents, this is a feared complication after esophageal surgery. Early detection of anastomotic leakage is crucial, as delayed start of treatment, which for example can result in mediastinitis and empyema, is associated with prolonged hospital stay, morbidity, and mortality (4,5).
A standardized postoperative recovery program can be helpful in early detection of complications and thereby improving the outcome after esophagectomy. Enhanced recovery after surgery (ERAS) pathways are designed to optimize perioperative care by a multimodal approach to the surgical patient (6). However, the optimal management strategies for detection of anastomotic leakages after esophagectomy are unclear (7,8). This study aims to review the available literature on the postoperative management after an esophagectomy including strategies to early recognize and detect anastomotic leakage and reflect on this from our experience.
Esophagectomy ERAS pathway UMCU
Esophagectomy
In our institute we routinely perform a fully robot-assisted minimally invasive esophagectomy (RAMIE). This includes a two-field lymphadenectomy (including mediastinal stations 2–9), gastric conduit reconstruction, and an intrathoracic anastomosis (Ivor Lewis) or a cervical anastomosis (McKeown). In our hospital, we perform a three-stage esophagectomy with a cervical anastomoses if indicated, for example in patients with an upper esophageal tumor, a Barrett’s segment extending into the upper esophagus, neoadjuvant radiation of the upper esophagus, or if a cervical lymph node dissection is indicated. Otherwise, for example in patients with a lower esophageal tumor we perform a two-stage esophagectomy with an intrathoracic anastomosis. The technical steps of RAMIE procedure were described in detail in previous publications (9). During the postoperative period patients will follow a standardized ERAS protocol.
Postoperative monitoring
During the postoperative time, the patient will be closely monitored to look for signs of complications. This close monitoring will first take place at the intensive care unit (day 0 postoperative), medium care (day 1 postoperative), and the regular upper GI surgical ward (day 2 postoperative). Specific manifestations include swelling and erythema of the cervical wound, in case of a cervical anastomosis, or change of the chest drain output, in case of an intra-thoracic anastomosis. Also tachycardia, new-onset atrial fibrillation (AF) and fever are alarm symptoms for possible complications (10). Other signs of sepsis related to mediastinitis such as subcutaneous emphysema, thoracic pain, pneumothorax, respiratory failure, or pleural effusion should warrant active further investigation.
During the first 3 days postoperatively laboratory diagnostics will be performed routinely. This will consist of infection parameters such as leucocyte count and C-reactive protein (CRP) as well as electrolytes, glucose, and hemoglobin. If alarm symptoms are present immediate start of supportive care by means of IV fluids, antibiotics, oxygen, and transfer to a high care unit should be considered, depending on the clinical status of the patient. A computed tomography (CT) of both chest and abdomen will be performed to rule out an anastomotic leakage or other complications. If the CT scan is inconclusive an endoscopic examination will be performed to visualize the anastomosis as well as the gastric conduit.
Decompressive nasogastric tube
After esophagectomy, in which a gastric conduit is used to restore gastro-intestinal continuity, a decompressive nasogastric tube is often used. The routinely use of a decompressive nasogastric tube postoperatively is debatable. A meta-analysis showed that perioperative or early removal of the nasogastric tube did not result in different rate of postoperative complications such as anastomotic leakage (11). Therefore, several units omitted the routine use of a nasogastric tube. However, in our institution we routinely use a nasogastric tube to reduce fluid accumulation and gastric distension. We use a 14 or 16 French nasogastric tube which is inserted shortly after start of surgery. During creation of the anastomoses the nasogastric tube is positioned at about 10 centimeters below the anastomoses and attached with a Bridle (Applied Medical Technology, Ohio, USA) nasal tube retaining system. This nasogastric tube will be in situ till day 4 postoperatively. We routinely perform a contrast swallow examination at day 4 postoperatively. This study examines both the swallow function of the patient, considering potential laryngeal recurrent nerve damage, and observes the emptying of the gastric tube, regarding potential delayed gastric emptying (Figure 1). To accurately observe the swallow function of the patient this study is performed under supervision of a speech therapist. The contrast swallow study is not primary performed to diagnose or rule out an anastomotic leakage.
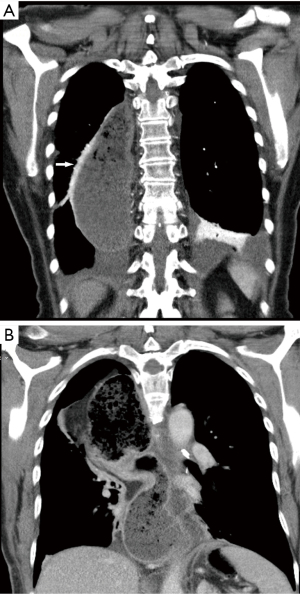
Average day to start oral feeding
After removal of the nasogastric tube patient will start oral intake containing water and clear fluids at day 4 postoperatively. The next day, a soft and pureed diet will be administered, which is maintained till the patient’s first visit to the outpatient clinic postoperatively, which is routinely planned at about 14 days after surgery.
In our institute we routinely place a percutaneous jejunostomy at the end of the abdominal phase of the esophagectomy. Main goal of this feeding tube is to realize adequate nutritional intake during the period a patient needs to get used to the new feeding regimen after the esophagectomy. Earlier, it was demonstrated that following an esophagectomy about 40% of the patients required nutritional intervention by nasojejunal tube or total parenteral nutrition if oral intake was started early postoperative and a jejunostomy was omitted during the esophagectomy (12). However, several upper GI units start oral feeding directly after an esophagectomy (13). Recently, a randomized controlled NUTRIENT-2 trial comparing direct start of oral feeding after an esophagectomy with a nil-by-mouth regimen for 5 days postoperative revealed that direct oral feeding after esophagectomy did not increase incidence of complications such as anastomotic leakage and pneumonia (14). In line with this, several upper GI units omit the use of a percutaneous jejunostomy as they start oral intake immediately postoperatively. However, the debate whether a percutaneous feeding jejunostomy is necessary is still ongoing. A recent study which evaluated the use of an jejunostomy in patients undergoing esophagectomy demonstrated that omitting percutaneous feeding jejunostomy placement was associated with prolonged hospital stay, higher in-hospital mortality and 30-day mortality (15). Another argument for tube placement is the expected weight loss that patients will suffer in the months following esophagectomy (16). However, the routine use of a percutaneous jejunostomy is related with serious complications. For example, the earlier mentioned Nutrient 2 trial for example showed in 7.5% of the patients a jejunostomy related complication. A retrospective study from our unit showed jejunostomy-related problems like luxation, occlusion, or reoperation in 31% of the patients (16).
Depending on the recovery of the patient we tend to remove the jejunostomy at about 8 weeks postoperatively. However, if an anastomotic leakage has been diagnosed, patient will be nil by mouth for an undefined period. During this period the patient will receive adequate nutritional intake administered through the jejunostomy, under the careful supervision of a dietician.
Early recognition of complications
Clinical symptoms
Early diagnose and treatment of postoperative complications is essential and may improve patient survival. During the postoperative time patients will be closely monitored to look for signs of complications. Specific manifestations of an anastomotic leak include a change of the chest drain output or swelling and erythema of the cervical wound. This immediately warrants both treatment, for example opening of the cervical wound or antibiotic treatment, and CT of both chest and abdomen to visualize possible thoracic involvement. If an anastomotic leakage occurs following a transthoracic esophagectomy with a cervical anastomoses, 60% of these patients developed an intrathoracic manifestation while in about 40% of the patients the leakage was confined to the neck (7). Therefore, awareness of possible intrathoracic spread of leakage of a cervical anastomoses is important for early recognition.
New-onset AF, fever, or extensive pain are alarm symptoms which warrant further examination. For example, new-onset AF is frequently observed after esophagectomy and may predict postoperative complications (17). Recently, it was demonstrated that new-onset AF is highly associated with post-operative complications like pneumonia and anastomotic leakage (10). Since AF seldomly occurs without other postoperative complications, further investigation (e.g., laboratory diagnostics, chest X-ray or CT scan) to evaluate patients with new-onset AF is recommended.
Laboratory diagnostics
Routine laboratory tests, in particular CRP and leukocytes, are used to monitor patients postoperatively and to screen for postoperative complications, such as anastomotic leakage. CRP is an acute-phase protein that is synthesized in hepatocytes in response to pro-inflammatory cytokines (18). CRP is secreted into the blood in response to inflammation and infection. Postoperative CRP is a predictor for postoperative complications, such as anastomotic leak, after esophagectomy (19). Several studies determined the relation between postoperative CRP increase and postoperative complications. For example, an elevated CRP (>177 mg/L) at day 2 postoperatively was shown to have a sensitivity of 0.9 and a specificity of 0.95 for anastomotic leakage after radical gastrectomy with esophageal-jejunum reconstruction (20). More recently, in patients who underwent a thoraco-abdominal esophagectomy a CRP increase on the second postoperative day above 200 mg/L was an independent positive predictor for postoperative complications (21). In conclusion, routine laboratory tests postoperatively have diagnostic value for anastomotic leakage. Early elevated pro-inflammatory markers warrants careful physical examination and further diagnostics test to detect possible complications after esophagectomy. However, results from randomized controlled trials are needed to exactly determine the role of inflammatory markers on outcome and prognosis. New markers, such as procalcitonin, septicyte and others are promising and warrant further evaluation (22).
Radiology
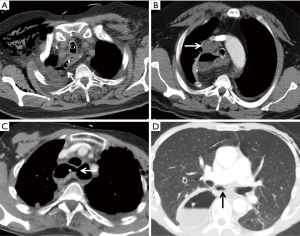
A CT scan is often performed to diagnose a postoperative anastomotic leakage, since it is non-invasive and safe to use in critically ill patients. In addition, it also aids in the detection of other associated findings such as pulmonary complications. Several studies have evaluated the efficacy of CT scanning for the detection of anastomotic leakage after esophagectomy, for example compared with contrast swallow examination, and showed better results with a CT scan for diagnosing an anastomotic leakage (23,24). Recently, our study group demonstrated that the presence of mediastinal fluid, mediastinal air, esophagogastric wall discontinuity and a fistula on a postoperative CT are strongly associated with anastomotic leakage after esophagectomy in patients with a clinical suspicion of anastomotic leakage (Figure 2) (25). In addition, a CT-based prediction score was developed for the detection of anastomotic leakage after esophagectomy. However, it can be difficult to confirm the significance of small pockets of fluid and air located in the mediastinum when CT scanning is performed in the first week postoperative. If the results of the CT are doubtful, an endoscopy for further analysis is recommended.
Contrast swallow examination
Worldwide, a water-soluble contrast swallow is routinely carried out around day 4 to 7 postoperatively to detect anastomotic leakage. However, limitation of routine contrast swallow in the detection of anastomotic leakage is the poor sensitivity. Our group demonstrated that a contrast swallow performed around day 7 postoperative had low sensitivity and low predictive value in patients with a cervical anastomoses (26). In addition, at this time-point over 50% of the patients with an anastomotic leakage already had clinically presentation, so before the contrast swallow examination was routinely planned. This is in line with other studies which also showed a poor sensitivity of contrast swallow examination to detect anastomotic leakage (27,28). A prospective trial that compared the accuracy of CT with oral contrast, endoscopy and contrast swallow for the detection of anastomotic leakage following esophagogastric surgery, concluded that a CT scan is more reliable compared with a contrast swallow to detect an anastomotic leak after an esophagectomy (24).
Endoscopy
Historically, surgeons have been reluctant to utilize endoscopic examination early after esophagectomy as this invasive procedure may damage the anastomosis. However, endoscopy has proven to be an accurate method to diagnose anastomotic leakage after esophagectomy (29). Thereby, an international survey showed that routinely performed endoscopy postoperatively is used by several surgeons (30). A study comparing endoscopy with CT in identifying anastomotic leakage after esophagectomy showed a more accurate identification of anastomotic leak than CT (31). Besides identification of the presence and location of leaks an endoscopy also provides more precise information on the condition of the gastric conduit. However, it provides no information about the mediastinal involvement, for which a CT scan is recommended. In our institute we first perform a CT scan to detect a possible anastomotic leakage. If inconclusive or if further treatment is warranted, we will proceed to perform an endoscopy. This can facilitate an immediate treatment of anastomotic leakage, for example by placement of an endoscopic endoluminal vacuum therapy, a nasogastric tube through the defect, or placement of an esophageal stent.
Conclusions
Esophagectomy with en-bloc lymphadenectomy after neoadjuvant chemo(radio)therapy is the standard of care for resectable locally advanced esophageal cancer. Postoperative complications may have a significant impact on the duration of hospital stay and quality of life. Intensive monitoring of the patient as part of a standardized ERAS program can help in early detection of complications, such as anastomotic leakage. In addition, an aggressive approach should be applied to both diagnose and treat anastomotic leakage. This may reduce failure to rescue rates and improve postoperative outcomes. However, an international guideline on the diagnostic and therapeutic management of anastomotic leakage is warranted.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Alejandro Nieponice) for the series “Anastomotic Techniques for Minimally Invasive Esophagectomy and Endoscopic Handling of Its Complications” published in Annals of Esophagus. The article has undergone external peer review.
Peer Review File: Available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-10/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-10/coif). The series “Anastomotic Techniques for Minimally Invasive Esophagectomy and Endoscopic Handling of Its Complications” was commissioned by the editorial office without any funding or sponsorship. RvH reports personal fees from Intuitive Inc., outside the submitted work. JPR reports personal fees from Intuitive Inc., outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shapiro J, van Lanschot JJB, Hulshof MCCM, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. [Crossref] [PubMed]
- Low DE, Kuppusamy MK, Alderson D, et al. Benchmarking complications associated with esophagectomy. Ann Surg 2019;269:291-8. [Crossref] [PubMed]
- Kim RH, Takabe K. Methods of esophagogastric anastomoses following esophagectomy for cancer: A systematic review. J Surg Oncol 2010;101:527-33. [Crossref] [PubMed]
- Goense L, Meziani J, Ruurda JP, et al. Impact of postoperative complications on outcomes after oesophagectomy for cancer. Br J Surg 2019;106:111-9. [Crossref] [PubMed]
- Kassis ES, Kosinski AS, Ross P Jr, et al. Predictors of anastomotic leak after esophagectomy: an analysis of the society of thoracic surgeons general thoracic database. Ann Thorac Surg 2013;96:1919-26. [Crossref] [PubMed]
- Findlay JM, Gillies RS, Millo J, et al. Enhanced recovery for esophagectomy: a systematic review and evidence-based guidelines. Ann Surg 2014;259:413-31. [Crossref] [PubMed]
- van Rossum PSN, Haverkamp L, Carvello M, et al. Management and outcome of cervical versus intrathoracic manifestation of cervical anastomotic leakage after transthoracic esophagectomy for cancer. Dis Esophagus 2017;30:1-8. [PubMed]
- van Heijl M, van Wijngaarden AK, Lagarde SM, et al. Intrathoracic manifestations of cervical anastomotic leaks after transhiatal and transthoracic oesophagectomy. Br J Surg 2010;97:726-31. [Crossref] [PubMed]
- van der Sluis PC, van der Horst S, May AM, et al. Robot-assisted minimally invasive thoracolaparoscopic esophagectomy versus open transthoracic esophagectomy for resectable esophageal cancer: a randomized controlled trial. Ann Surg 2019;269:621-30. [Crossref] [PubMed]
- Seesing MFJ, Scheijmans JCG, Borggreve AS, et al. The predictive value of new-onset atrial fibrillation on postoperative morbidity after esophagectomy. Dis Esophagus 2018; [Crossref] [PubMed]
- Weijs TJ, Kumagai K, Berkelmans GH, et al. Nasogastric decompression following esophagectomy: a systematic literature review and meta-analysis. Dis Esophagus 2017;30:1-8. [PubMed]
- Weijs TJ, Berkelmans GH, Nieuwenhuijzen GA, et al. Immediate postoperative oral nutrition following esophagectomy: a multicenter clinical trial. Ann Thorac Surg 2016;102:1141-8. [Crossref] [PubMed]
- Cheong E. How minimally invasive esophagectomy was implemented at the Norfolk and Norwich University Hospital. J Thorac Dis 2017;9:S879-85. [Crossref] [PubMed]
- Berkelmans GHK, Fransen LFC, Dolmans-Zwartjes ACP, et al. Direct oral feeding following minimally invasive esophagectomy (NUTRIENT II trial): an international, multicenter, open-label randomized controlled trial. Ann Surg 2020;271:41-7. [Crossref] [PubMed]
- Watson M, Trufan S, Benbow JH, et al. Jejunostomy at the time of esophagectomy is associated with improved short-term perioperative outcomes: analysis of the NSQIP database. J Gastrointest Oncol 2020;11:421-30. [Crossref] [PubMed]
- Weijs TJ, van Eden HWJ, Ruurda JP, et al. Routine jejunostomy tube feeding following esophagectomy. J Thorac Dis 2017;9:S851-60. [Crossref] [PubMed]
- Seesing MFJ, Borggreve AS, Ruurda JP, et al. New-onset atrial fibrillation after esophagectomy for cancer. J Thorac Dis 2019;11:S831-4. [Crossref] [PubMed]
- Du Clos TW. Function of C-reactive protein. Ann Med 2000;32:274-8. [Crossref] [PubMed]
- Gordon AC, Cross AJ, Foo EW, et al. C-reactive protein is a useful negative predictor of anastomotic leak in oesophago-gastric resection. ANZ J Surg 2018;88:223-7. [Crossref] [PubMed]
- Ji L, Wang T, Tian L, et al. The early diagnostic value of C-reactive protein for anastomotic leakage post radical gastrectomy for esophagogastric junction carcinoma: a retrospective study of 97 patients. Int J Surg 2016;27:182-6. [Crossref] [PubMed]
- Babic B, Tagkalos E, Gockel I, et al. C-reactive protein levels after esophagectomy are associated with increased surgical trauma and complications. Ann Thorac Surg 2020;109:1574-83. [Crossref] [PubMed]
- Verboom DM, Koster-Brouwer ME, Ruurda JP, et al. A pilot study of a novel molecular host response assay to diagnose infection in patients after high-risk gastro-intestinal surgery. J Crit Care 2019;54:83-7. [Crossref] [PubMed]
- Hogan BA, Winter DC, Broe D, et al. Prospective trial comparing contrast swallow, computed tomography and endoscopy to identify anastomotic leak following oesophagogastric surgery. Surg Endosc 2008;22:767-71. Erratum in: Surg Endosc 2008;22:2329. [Crossref] [PubMed]
- Strauss C, Mal F, Perniceni T, et al. Computed tomography versus water-soluble contrast swallow in the detection of intrathoracic anastomotic leak complicating esophagogastrectomy (Ivor Lewis): a prospective study in 97 patients. Ann Surg 2010;251:647-51. [Crossref] [PubMed]
- Goense L, Stassen PMC, Wessels FJ, et al. Diagnostic performance of a CT-based scoring system for diagnosis of anastomotic leakage after esophagectomy: comparison with subjective CT assessment. Eur Radiol 2017;27:4426-34. [Crossref] [PubMed]
- Boone J, Rinkes IB, van Leeuwen M, et al. Diagnostic value of routine aqueous contrast swallow examination after oesophagectomy for detecting leakage of the cervical oesophagogastric anastomosis. ANZ J Surg 2008;78:784-90. [Crossref] [PubMed]
- Hu Z, Wang X, An X, et al. The diagnostic value of routine contrast esophagram in anastomotic leaks after esophagectomy. World J Surg 2017;41:2062-7. [Crossref] [PubMed]
- Tirnaksiz MB, Deschamps C, Allen MS, et al. Effectiveness of screening aqueous contrast swallow in detecting clinically significant anastomotic leaks after esophagectomy. Eur Surg Res 2005;37:123-8. [Crossref] [PubMed]
- Page RD, Asmat A, McShane J, et al. Routine endoscopy to detect anastomotic leakage after esophagectomy. Ann Thorac Surg 2013;95:292-8. [Crossref] [PubMed]
- Hagens ERC, Anderegg MCJ, van Berge Henegouwen MI, et al. International survey on the management of anastomotic leakage after esophageal resection. Ann Thorac Surg 2018;106:1702-8. [Crossref] [PubMed]
- Song P, Li J, Zhang Q, et al. Ultrathin endoscopy versus computed tomography in the detection of anastomotic leak in the early period after esophagectomy. Surg Oncol 2020;32:30-4. [Crossref] [PubMed]
Cite this article as: van den Berg JW, van der Horst S, van Hillegersberg R, Ruurda JP. Enhanced recovery after surgery pathway in esophagectomy in a high volume center: clinical keys to early leak diagnosis after esophagectomy. Ann Esophagus 2022;5:14.


