
Minimally invasive Ivor-Lewis esophagectomy with linear stapled side-to-side anastomosis
Introduction
Improvements in perioperative care, such as introduction of minimally invasive surgery and enhanced recovery after surgery programs (ERAS), have significantly reduced postoperative morbidity and improved recovery (1-3). However, postoperative morbidity remains substantial and anastomotic leakage (AL) remains one of the most detrimental complications with a significant impact on quality of life and it is even associated with increased cancer recurrence and reduced long-term survival rates (3). Several aspects of perioperative care are important to reduce AL rate. A dedicated team of experienced surgeons, anesthesiologists, scrub nurses, intensivists, paramedics and nurse specialists need to be involved in the entire care pathway to provide optimal care. It has previously been shown, that the learning curve plays an important role in postoperative morbidity, especially for AL (4,5).
The anastomotic techniques in minimally invasive Ivor-Lewis esophagectomy (MIE-IL) vary from a hand sewn (HS) technique, a circular stapling (CS) technique, or a semi-mechanical side-to-side linear stapling (LS) technique. At our institution, the LS technique has been implemented since 2012 as data suggested that AL and anastomotic stricture rates were lower with a LS technique (6-9). Furthermore, the LS technique was already used for the bariatric and gastric cancer surgery at our institution. It has been optimized during the years and is standardized since 2016.
The aim of this study is to describe the linear stapled side-to-side anastomotic technique in patients undergoing a MIE-IL and report on the postoperative outcomes of patients operated with this technique. We present the following article in accordance with the STROBE reporting checklist (available at https://aoe.amegroups.com/article/view/10.21037/aoe-20-97/rc).
Methods
Study design
This single-center cohort study was conducted at the Catharina Hospital, Eindhoven, The Netherlands. All patients aged 18 or above that underwent a MIE-IL with linear stapled side-to-side anastomosis, between January 2016 and November 2020 at the Catharina Hospital Eindhoven, the Netherlands, were eligible for inclusion in this study. There were no exclusion criteria.
Data were retrospectively collected and analyzed without patient identifiers. Informed consent was not obtained due to the nature of the study. However, patients that previously explicitly indicated their data were not to be used for research or educational purposes, would be excluded.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Medical Ethics Committee (W20.314) and institutional review board of the Catharina Hospital (nWMO-2021.001). Data were retrospectively collected and analyzed without patient identifiers. Informed consent was not obtained due to the nature of the study. However, patients that previously explicitly indicated their data were not to be used for research or educational purposes would be excluded.
Definitions
Postoperative complications were classified according to the Clavien-Dindo classification of surgical complications (10). Cardiac complications included: cardiac arrest, cardiac ischemia/infarction, pericarditis, congestive heart failure and a-/dysrhythmias requiring intervention. Pulmonary complications included: (aspiration)pneumonia, pleural effusion/empyema, pneumothorax and atelectasis requiring intervention and acute respiratory distress syndrome (ARDS) and respiratory insufficiency requiring prolonged treatment or reintubation. Pneumonia was scored using the Uniform Pneumonia Score (UPS) (11). AL was scored using the Esophagectomy Complications Consensus Group (ECCG) definition (12). Anastomotic stricture was defined as symptomatic dysphagia due to a stenosis that required endoscopic dilation. Pathological tumor stage (pTNM) was classified using the 8th edition of the Union for International Cancer Control (UICC).
Statistical analyses
After assessment of data normality (assumed normal if both skewness and kurtosis ranges were between −1 to 1), data were presented as means with standard deviation (SD) or medians with its interquartile range (IQR). Absolute numbers were presented with their corresponding percentage.
Surgical technique
Laparoscopic phase
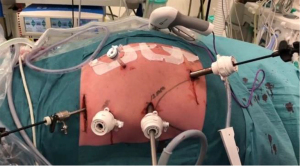
After induction of general anesthesia and intubation with a single lumen endotracheal tube, the patient is placed in supine split leg position (French position) and reverse Trendelenburg. The operating surgeon stands between the legs, the assistant surgeon on the left and the scrub nurse on the right side of the patient. Five abdominal ports are inserted (Figure 1). Primary access is gained via a 12 mm camera port that is placed above the umbilicus (lower third between the xiphoid process and the umbilicus) and to the left of the abdominal midline, after insufflation of the abdomen with a Veress needle at Palmers’ point. In our experience, this positioning of trocars provides a better laparoscopic vision/exposure and is more ergonomic. Intra-abdominal pressure is maintained at 14 mmHg. All additional ports are inserted under direct laparoscopic vision. Two 12 mm ports are inserted, one left and one right of the abdominal midline (one hand width) and slightly cranial to the camera port. One 5 mm port is inserted in the right anterior axillary line slightly below the subcostal margin and finally, a 5 mm Nathanson liver retractor is inserted slightly below the xiphoid. Once the ports are placed, the abdomen is inspected first to rule out advanced/metastatic disease of the peritoneal surfaces and liver. After inspection, the greater curvature of the stomach is mobilized using a no-touch technique. A window is created in the gastrocolic omentum and dissection is performed along the greater curve of the stomach with continuous care for the right gastroepiploic artery to ensure adequate vascularization of the gastroepiploic arcade. Near the watershed an omental patch is created to cover the future intrathoracic anastomosis after construction (Figure 2). Subsequently, the short gastric vessels and gastrosplenic ligament are divided and coagulated using a sealing device, until the left lateral part of the greater curvature and left crus of the diaphragm are dissected free. After the greater curvature is mobilized, a limited Kocher’s maneuver is performed and the stomach is considered adequately mobilized if the pylorus can reach the right crus without tension. Next, the lesser omentum is opened at the antropyloric area thereby exposing the caudate lobe of the liver. The right gastric artery is divided slightly below the terminal branches of the vagal trunk (crow’s foot). A complete D2 lymphadenectomy is routinely performed from the liver hilum along the celiac axis toward the splenic hilum (Figure 3). The retrogastric attachments are dissected free and the left gastric artery/vein pedicle is identified and divided after placement of Hem-o-lok® clips. The gastric conduit is now measured at 4.5 cm from the greater curvature using the felt tip of a marker pen (Figure 4). The gastric conduit is created using the Endo-GIA™ Tri-Staple™ XL with 45 mm purple staples. Using near infrared spectroscopy (NIRS), the watershed area of the gastroepiploic arteries is identified and the gastric conduit is transected at the level of the well-perfused part of the conduit (Figure 5). A pyloroplasty is not routinely performed.
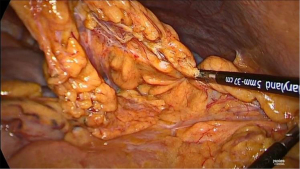
Once the gastric conduit is created, the distal thoracic esophagus is dissected free transhiatally. An en-bloc para-esophageal lymphadenectomy is performed toward the pulmonary vein by opening the pleural cavities and dividing both inferior pulmonary ligaments. During this phase, a small pleural drain is placed in the left pleural cavity to prevent a tension pneumothorax. A cruroplasty is performed using a Ti-Cron™ 0 suture with SK-1 needle. To ensure adequate mobilization of the gastric conduit during the thoracoscopic phase, the hiatus is not closed during the abdominal phase, but the suture is placed in the right pleural cavity for future closing in the thorax. The staple line crossings on the gastric conduit are reinforced with Biosyn™ 3-0 sutures and the gastric conduit is fixed to the specimen and placed in the left pleural cavity. Finally, a jejunostomy is constructed in the left lower quadrant. An extra 12 mm port is inserted in the right hypogastric region and the laparoscope is switched to the 12 mm right para-umbilical port. The ligament of Treitz is identified and after tracing the jejunum it is fixed to the abdominal wall with three triangularly placed sutures and an anti-rotation suture. The feeding tube is now passed into the jejunum under direct laparoscopic vision using the Seldinger technique. After final inspection of the abdomen the ports are removed under direct laparoscopic vision and the fascia and skin incisions are closed.
Thoracoscopic phase
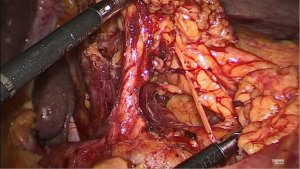
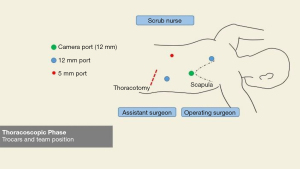
For the thoracoscopic phase and creation of the intrathoracic anastomosis, a 34 Charrière gastric tube is inserted via the mouth by the anesthesiologist and the patient is placed in prone position with the single lumen endotracheal tube still in position. Two cushions are placed under the proximal thorax and the pelvis rests on one cushion ensuring that the abdomen is free to prevent abdominal pressure that can move the diaphragm cranially during the procedure and thereby limit the working space and view. The operating surgeon stands on the right side of the patient, as well as the assistant surgeon (who stands left of the operating surgeon) and the scrub nurse stands on the left side of the patient (opposite to the surgeons). Four thoracic ports are placed in the right thorax (Figure 6). A 12 mm camera port is inserted just inferior to the tip of the scapula. Another 12 mm port is inserted cranially at the medial margin of the scapula, a 5 mm port caudally and paravertebral and finally a 12 mm port is inserted more caudally and in line with the lower tip of the scapula and camera port. Intrathoracic/pleural pressure is maintained at 8 mmHg. Mobilization of the thoracic esophagus begins by further dividing the inferior pulmonary ligament and performing an en-bloc lymphadenectomy of the para-esophageal, infracarinal nodes and those in the aortopulmonary window. First the mediastinal pleura covering the esophagus is incised and divided toward the azygos vein. Once the azygos vein is isolated it is divided with a 30 mm vascular cartridge using the Endo-GIA™ Tri-Staple™. The mediastinal pleura above the azygos vein is preserved. The pulmonary branches of the vagus nerve that course to the right main bronchus and supply the right lung are dissected and spared since we hypothesize that preserving these branches may reduce postoperative pulmonary complications (13). A vagotomy is performed distal to the pulmonary branches. The esophagus is now dissected from the trachea exposing the left mainstem bronchus and this is continued cranially over the trachea to ensure adequate space for the gastric conduit. Attention is then turned to further mobilizing the esophagus by dissecting the esophagus and para-esophageal lymph nodes circumferentially. Finally, the subcarinal lymph nodes are harvested. In patients with squamous cell carcinoma or preoperatively diagnosed pathological lymph nodes at this site, an extended lymphadenectomy is carefully performed to include lymph nodes along the aortopulmonary window and paratracheally, while preserving the vagus and recurrent nerves. To prevent the occurrence of a chylothorax, the proximal intrathoracic portion of the thoracic duct is identified and routinely clipped with a Hem-o-lok® clip and resected with the specimen. Next, the esophagus is transected proximally above the azygos vein with a 60 mm purple staple cartridge using the Endo-GIA™ Tri-Staple™. A stay suture is placed at one third of the staple line. While keeping tension on the stay suture, the staple line is now cut from right to left toward this suture using a diathermy to create a flap for manipulation of the proximal esophagus during the creation of the anastomosis. Subsequently a second stay suture is placed. To ensure that the muscular layer is not retracted cranially during further handling of the anastomosis, the mucosa is fixed to the muscular layer at three additional points using Biosyn™ 3-0 (Figure 7).
Anastomosis
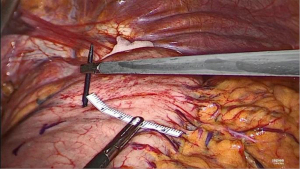
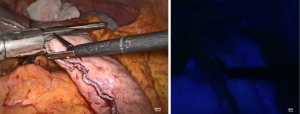
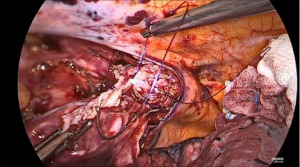
Prior to performing the anastomosis, the 34 Charrière gastric tube is advanced exiting the proximal esophagus. Now, the suture between the specimen and previously constructed gastric conduit is cut and the gastric conduit is carefully mobilized toward the thoracic cavity and proximal esophagus to create the anastomosis. A no touch technique is utilized as much as possible by carefully moving the omental patch and gastroepiploic pedicle, and thereby indirectly mobilizing the gastric conduit. At 5 cm from the top of the gastric conduit and as close to the vascular pedicle as possible, a small incision is made using a diathermy device (Figure 8). Subsequently, the Endo-GIA™ XL Tri-Staple™ with a 30 mm purple cartridge is inserted with its anvil in the gastric conduit. If the gastric conduit and the proximal esophagus are adequately mobilized it should be easily brought to the level of the proximal esophagus and without tension. The 34 Charrière gastric tube is then retracted by the anesthesiologist and the tip of the stapler is advanced into the lumen of the proximal esophagus while keeping tension on the previously placed stay suture to facilitate its complete insertion (Figure 9). The side-to-side anastomosis is created after verification that the 34 Charrière gastric tube is free from the stapler. Now, the 34 Charrière gastric tube is advanced and moved further into the gastric conduit. The defect is closed with two V-Loc™ 4.0 sutures starting on the left side (Figure 10). The flap of the previously (partially) incised staple line of the proximal esophagus is used for manipulation for adequate exposure of the defect from all sides. Once the first V-Loc™ has reached the initial transection, the flap is cut and removed. A second V-Loc™ is then used to close the remaining defect from the other side. The 34 Charrière gastric tube is withdrawn proximally of the anastomosis and the left V-Loc™ is partially used for a second layer of inverted sutures. Subsequently, four to five inverting horizontal mattress sutures (Biosyn™ 4-0) are placed from the right side. The integrity of the anastomosis is tested with a methylene blue insufflation test through the 34 Charrière gastric tube which is removed after the test.
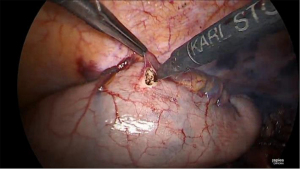
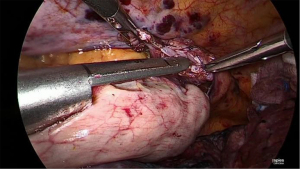
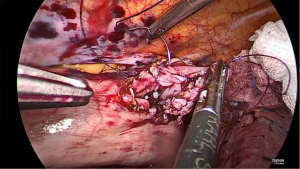
Final stage
Once the side-to-side anastomosis is completed (Figure 11), the omental patch from the greater curve is wrapped around the anastomosis and advanced into the pocket of the proximal mediastinal pleura between the gastric conduit, proximal esophagus and the trachea (Figure 12). The previous cruroplasty is now closed with the Ticron™ 0 suture using a knot pusher. The specimen is placed in an endoscopic pouch with memory wire. A small thoracotomy of 5–6 cm is made as caudally as possible (Figure 13), since there is more intercostal space at this site. The pleura is left intact in order to maintain the intrathoracic pressure so that a 5 mm port can be inserted under direct thoracoscopic vision to facilitate moving the endoscopic pouch towards the thoracotomy. A Jackson-Pratt® drain is then placed on the omental patch and a small pleural drain is left in the right pleural cavity. Finally, the thoracotomy is opened and the endoscopic pouch with specimen is retrieved. After final inspection of the thoracic cavity, the ports are removed under direct thoracoscopic vision and the fascia and skin incisions are closed.
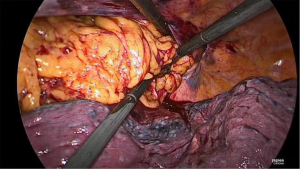
Postoperative management
Patients are extubated directly postoperatively on the operating room and are transferred to the ICU. The next day patients are routinely transferred to a specialized surgical unit. No nasogastric decompression is used postoperatively (14). Pleural drains are removed on postoperative day one (POD1) based on assessment on X-ray imaging. Patients routinely receive standardized analgesics via either a paravertebral catheter or thoracic epidural, in combination with intravenous patient-controlled analgesia (morphine PCA). The Jackson-Pratt® drain is left within the chest cavity to monitor amylase levels on a daily basis and this drain is routinely removed at POD4. All patients receive postoperative care according to a standardized ERAS program. Patients directly start with (liquid) oral feeding from POD1 and this is gradually expanded to an unlimited amount of liquid foods from POD6 and solid foods from POD15. This nutritional protocol has been implemented since 2018 and has been shown to improve functional recovery and reduce length of hospital stay (LOHS), while it does not affect AL or pulmonary (pneumonia) complication rate (1,2).
Results
Baseline characteristics
A total of 246 patients that underwent a minimally invasive esophagectomy with intrathoracic LS side-to-side anastomosis for locally advanced esophageal carcinoma, between January 2016 and November 2020, were eligible for inclusion. All patients were included in the study. Mean age was 65 years (SD ±9). Comorbidities were present in 66.7% of patients as shown in Table 1. Median BMI was 25.6 kg/m2 (IQR 23.3–28.4 kg/m2). Most patients (71.1%) were American Society of Anesthesiologists (ASA) grade II or grade III (26.0%). Adenocarcinoma was the predominant histological subtype in 84.1% of patients, as opposed to 13.4% of patients with squamous-cell carcinoma. A total of 222 patients (90.2%) underwent neoadjuvant chemoradiotherapy. Mean duration of surgery was 249 (SD ±36) minutes and surgical conversion rate was 0.8% (n=2). Median intraoperative blood loss was 100 mL (IQR 100–200 mL).
Table 1
| Characteristics | Total cohort (n=246) |
|---|---|
| Age at inclusion, years | 65 [8.9] |
| Gender, male | 195 (79.3) |
| BMI at diagnosis, kg/m2 | 25.6 [23.3–28.4] |
| Weight loss, kg | 3.0 [0–6.0] |
| ASA classification | |
| I | 5 (2.0) |
| II | 175 (71.1) |
| III | 64 (26.0) |
| IV | 2 (0.8) |
| Comorbidity | 164 (66.7) |
| Cardiac | 50 (20.3) |
| Pulmonary | 40 (16.3) |
| Vascular | 79 (32.1) |
| Diabetes | 26 (10.6) |
| Obesity (BMI >30) | 41 (16.7) |
| Histology | |
| Adenocarcinoma | 205 (83.3) |
| Squamous cell carcinoma | 35 (14.2) |
| Tumor location | |
| Mid | 16 (6.5) |
| Distal | 158 (64.2) |
| Gastroesophageal junction | 69 (28.0) |
| Neoadjuvant treatment | |
| None | 16 (6.5) |
| Chemoradiotherapy | 221 (89.8) |
| pTNM stage | |
| 0 | 55 (22.4) |
| I | 47 (19.1) |
| II | 39 (15.9) |
| III | 93 (37.8) |
| IV | 12 (4.9) |
| Intraoperative characteristics | |
| Duration of surgery, minutes | 250 [35] |
| Intraoperative complication | 8 (3.3) |
| Intraoperative blood loss, mL | 100 [100–200] |
Values are absolute numbers (percentage), means [standard deviation] or medians [lower quartile–upper quartile]. BMI, body mass index; ASA American Society of Anesthesiologists.
Postoperative outcomes
Thirty-day overall postoperative complication rate was 56.9%. Pneumonia rate (UPS) was 28.5%. The overall AL rate was 8.9% (n=22). In 11 cases (4.5%) these were minor leaks that could be managed conservatively (Type I leaks according to ECCG definition), whereas in 8 cases (3.3%) endoscopic intervention was required (Type II). In seven patients with a Type II leak, a fully covered self-expandable metal stent (SEMS; HANAROSTENT®) was placed. In one patient with a Type II leak, there were two separate leaks/cavities. These were managed by placing two 7F double pig-tail drains in one cavity and one 10F double pig-tail in the other cavity. Three patients (1.2%) required a reoperation (Type III). In these patients, the right side of the proximal gastric conduit, which is the most crucial site of the anastomosis, was ischemic. In two patients, the leak was covered by a SEMS, but thoracoscopic debridement was needed due to pleural/mediastinal contamination. In the other patient, the AL caused a fistula of the left mainstem bronchus that required thoracoscopic repair as well as revision of the anastomosis. Anastomotic stricture rate at 90 days postoperatively was 2.2% (n=5) in patients with at least 90 days of follow-up. In four patients, the anastomosis was dilated using a Savary dilation technique and in one patient a bio-degradable stent was required due to a twisted gastric conduit. Median LOHS was 8 days (IQR 7–12 days). Hospital readmission rate was 10.2%. Thirty-day mortality (including in-hospital mortality) rate was 1.6% (n=4). Of these, one patient died due to SARS-CoV-2. Perioperative outcomes are further subdivided for each year and presented in Table 2.
Table 2
| Variables | 2016 (n=49) | 2017 (n=61) | 2018 (n=49) | 2019 (n=43) | 2020 (n=44) |
|---|---|---|---|---|---|
| Length of hospital stay, days | 9 [7–16] | 9 [7–12] | 8 [7–10] | 7 [6–11] | 6 [6–7] |
| 30-day overall complications | 33 (67.3) | 41 (67.2) | 28 (57.1) | 21 (48.8) | 17 (38.6) |
| 30-day mortality* | 2 (4.1) | 0 | 0 | 0 | 2 (4.5) |
| Highest Clavien-Dindo grade | |||||
| Grade I | 3 (6.1) | 1 (1.6) | 1 (2.0) | 0 | 2 (4.5) |
| Grade II | 8 (16.3) | 21 (34.4) | 15 (30.6) | 7 (16.3) | 6 (13.6) |
| Grade IIIa | 7 (14.3) | 5 (8.2) | 7 (14.3) | 7 (16.3) | 4 (9.1) |
| Grade IIIb | 1 (2.0) | 1 (1.6) | 3 (6.1) | 2 (4.7) | 4 (9.1) |
| Grade IV | 10 (20.4) | 13 (21.3) | 2 (4.1) | 4 (9.3) | 1 (2.3) |
| Grade V | 2 (4.1) | 0 | 0 | 0 | 1 (2.3) |
| Pneumonia (UPS) | 18 (36.7) | 19 (31.1) | 18 (36.7) | 9 (20.9) | 6 (13.6) |
| Anastomotic leakage | 6 (12.2) | 5 (8.2) | 4 (8.2) | 4 (9.3) | 3 (6.8) |
| ECCG Type I | 3 | 0 | 3 | 3 | 2 |
| ECCG Type II | 2 | 4 | 1 | 1 | 0 |
| ECCG Type III | 1 | 1 | 0 | 0 | 1 |
| Size of defect | |||||
| <1.5 cm | 4 | 4 | 4 | 3 | 2 |
| >1.5 cm | 2 | 1 | 0 | 1 | 1 |
| Extent of tissue necrosis | |||||
| <2 cm or absent | 2 | 4 | 4 | 2 | 1 |
| >2 cm | 4 | 1 | 0 | 2 | 2 |
Values are absolute numbers (percentage) or medians [lower quartile–upper quartile]. * including in-hospital mortality. One patient in 2020 died due to SARS-CoV-2. UPS Uniform Pneumonia Score; ECCG Esophagectomy Complications Consensus Group.
Discussion
In this study, we report on postoperative outcomes after a MIE-IL with a LS side-to-side anastomotic technique since it was standardized in 2016 at our institution. In 2012, the LS technique—as part of the totally minimal invasive Ivor-Lewis esophagectomy—was implemented since a comparable technique was already used in bariatric and gastric cancer surgery at our institution. After several optimization steps, the technique as described in this study has been standardized since 2016.
The overall AL rate in our cohort was 8.9%. This was similar and possibly slightly lower than previous reports on the LS technique. However, comparison may be difficult, since the definition and incidence of AL after an esophagectomy is diverse and a clear definition for AL is missing in most studies (15-19). Interestingly, in the current cohort the AL rate further declined over the years even after the LS technique was standardized. A MIE-IL is a technically challenging procedure which is associated with a substantial learning curve and learning associated morbidity, particularly the occurrence of AL (4,5). The LS anastomotic technique further adds to the complexity of the MIE-IL as opposed to a HS end-to-end or circular CS end-to-side anastomosis, due to the technical difficulties in suturing the anterior defect thoracoscopically (15-17). Although this learning curve may hinder widespread adoption of the LS side-to-side technique, only the anterior portion of side-to-side anastomosis is hand-sewn as opposed to the HS end-to-end anastomotic technique, which may reduce leaks due to fewer technical imperfections (15-17).
Moreover, by performing the anastomosis in a side-to-side fashion, better vascularized parts of the gastric conduit and remaining proximal esophagus can be utilized for a wider anastomosis and a more triangulated lumen compared to the HS and CS (15-19). The staple line also reinforces the posterior wall and spreads the distribution of the shear forces on the anastomosis, which is associated with better functional results (e.g., less dysphagia, less benign anastomotic strictures requiring fewer dilatations and a lower incidence of recurrent laryngeal nerve injury) (15,16,19,20). Anastomotic stricture rate in our cohort was very low (2.2%) and previous studies have also reported a significantly lower incidence of anastomotic stricture after a LS side-to-side anastomosis due to the decreased tension and increased perfusion (15,16,19,20). In addition, AL is considered an important predisposing factor for stricture formation and the reduced AL rate may also explain the low stricture rate in our cohort (15).
This study was limited due to the retrospective and non-randomized design and by the fact that only a single expert center was included. Another limitation is that we could not compare our data to a historic cohort since data on patients that underwent surgery prior to implementation of the LS technique were not available. Although this center and team had already surpassed the learning curve, (minimal) learning associated morbidity and continuous improvements to the perioperative pathway (optimizing ERAS with direct start of oral feeding postoperatively and implementation of the PREPARE prehabilitation program) may be a potential source of bias as reflected by the reduction in postoperative morbidity and LOHS (1-3,21,22). The strength of this study is that patients were included from a high-volume center and all eligible patients could be included thereby limiting potential selection bias. Furthermore, most patient data were already collected prospectively for previous studies (1,2) and, in contrast to most studies, complications were scored according to predefined and validated definitions (10-12).
In conclusion, our results suggest that the minimally invasive esophagectomy with intrathoracic LS side-to-side esophagogastric anastomosis is safe and associated with a low rate of anastomotic complications and low mortality rate in a high-volume expert center.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Alejandro Nieponice) for the series “Anastomotic Techniques for Minimally Invasive Esophagectomy and Endoscopic Handling of Its Complications” published in Annals of Esophagus. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://aoe.amegroups.com/article/view/10.21037/aoe-20-97/rc
Data Sharing Statement: Available at https://aoe.amegroups.com/article/view/10.21037/aoe-20-97/dss
Peer Review File: Available at https://aoe.amegroups.com/article/view/10.21037/aoe-20-97/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoe.amegroups.com/article/view/10.21037/aoe-20-97/coif). The series “Anastomotic Techniques for Minimally Invasive Esophagectomy and Endoscopic Handling of Its Complications” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Medical Ethics Committee (W20.314) and institutional review board of the Catharina Hospital (nWMO-2021.001). Data were retrospectively collected and analyzed without patient identifiers. Informed consent was not obtained due to the nature of the study. However, patients that previously explicitly indicated their data were not to be used for research or educational purposes would be excluded.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fransen LFC, Janssen T, Aarnoudse M, et al. Direct Oral Feeding After a Minimally Invasive Esophagectomy: A Single-Center Prospective Cohort Study. Ann Surg 2020; [Crossref] [PubMed]
- Berkelmans GHK, Fransen LFC, Dolmans-Zwartjes ACP, et al. Direct Oral Feeding Following Minimally Invasive Esophagectomy (NUTRIENT II trial): An International, Multicenter, Open-label Randomized Controlled Trial. Ann Surg 2020;271:41-7. [Crossref] [PubMed]
- Fransen LFC, Luyer MDP. Effects of improving outcomes after esophagectomy on the short- and long-term: a review of literature. J Thorac Dis 2019;11:S845-S850. [Crossref] [PubMed]
- Claassen L, van Workum F, Rosman C. Learning curve and postoperative outcomes of minimally invasive esophagectomy. J Thorac Dis 2019;11:S777-S785. [Crossref] [PubMed]
- van Workum F, Stenstra MHBC, Berkelmans GHK, et al. Learning Curve and Associated Morbidity of Minimally Invasive Esophagectomy: A Retrospective Multicenter Study. Ann Surg 2019;269:88-94. [Crossref] [PubMed]
- Kondra J, Ong SR, Clifton J, et al. A change in clinical practice: a partially stapled cervical esophagogastric anastomosis reduces morbidity and improves functional outcome after esophagectomy for cancer. Dis Esophagus 2008;21:422-9. [Crossref] [PubMed]
- Raz DJ, Tedesco P, Herbella FA, et al. Side-to-side stapled intra-thoracic esophagogastric anastomosis reduces the incidence of leaks and stenosis. Dis Esophagus 2008;21:69-72. [Crossref] [PubMed]
- Orringer MB, Marshall B, Iannettoni MD. Eliminating the cervical esophagogastric anastomotic leak with a side-to-side stapled anastomosis. J Thorac Cardiovasc Surg 2000;119:277-88. [Crossref] [PubMed]
- Behzadi A, Nichols FC, Cassivi SD, et al. Esophagogastrectomy: the influence of stapled versus hand-sewn anastomosis on outcome. J Gastrointest Surg 2005;9:1031-40. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- van der Sluis PC, Verhage RJ, van der Horst S, et al. A new clinical scoring system to define pneumonia following esophagectomy for cancer. Dig Surg 2014;31:108-16. [Crossref] [PubMed]
- Low DE, Alderson D, Cecconello I, et al. International Consensus on Standardization of Data Collection for Complications Associated With Esophagectomy: Esophagectomy Complications Consensus Group (ECCG). Ann Surg 2015;262:286-94. [Crossref] [PubMed]
- Weijs TJ, Ruurda JP, Luyer MD, et al. Preserving the pulmonary vagus nerve branches during thoracoscopic esophagectomy. Surg Endosc 2016;30:3816-22. [Crossref] [PubMed]
- Weijs TJ, Kumagai K, Berkelmans GH, et al. Nasogastric decompression following esophagectomy: a systematic literature review and meta-analysis. Dis Esophagus 2017;30:1-8. [PubMed]
- Harustiak T, Pazdro A, Snajdauf M, et al. Anastomotic leak and stricture after hand-sewn versus linear-stapled intrathoracic oesophagogastric anastomosis: single-centre analysis of 415 oesophagectomies. Eur J Cardiothorac Surg 2016;49:1650-9. [Crossref] [PubMed]
- Price TN, Nichols FC, Harmsen WS, et al. A comprehensive review of anastomotic technique in 432 esophagectomies. Ann Thorac Surg 2013;95:1154-60. [Crossref] [PubMed]
- Wang F, Zhang H, Zheng Y, et al. Intrathoracic side-to-side esophagogastrostomy with a linear stapler and barbed suture in robot-assisted Ivor Lewis esophagectomy. J Surg Oncol 2019;120:1142-7. [Crossref] [PubMed]
- Gao HJ, Mu JW, Pan WM, et al. Totally mechanical linear stapled anastomosis for minimally invasive Ivor Lewis esophagectomy: Operative technique and short-term outcomes. Thorac Cancer 2020;11:769-76. [Crossref] [PubMed]
- Ben-David K, Tuttle R, Kukar M, et al. Minimally Invasive Esophagectomy Utilizing a Stapled Side-to-Side Anastomosis is Safe in the Western Patient Population. Ann Surg Oncol 2016;23:3056-62. [Crossref] [PubMed]
- van Workum F, van der Maas J, van den Wildenberg FJ, et al. Improved Functional Results After Minimally Invasive Esophagectomy: Intrathoracic Versus Cervical Anastomosis. Ann Thorac Surg 2017;103:267-73. [Crossref] [PubMed]
- Wynter-Blyth V, Moorthy K. Prehabilitation: preparing patients for surgery. BMJ 2017;358:j3702. [Crossref] [PubMed]
- Doganay E, Moorthy K. Prehabilitation for esophagectomy. J Thorac Dis 2019;11:S632-S638. [Crossref] [PubMed]
Cite this article as: Janssen HJB, Nieuwenhuijzen GAP, Luyer MDP. Minimally invasive Ivor-Lewis esophagectomy with linear stapled side-to-side anastomosis. Ann Esophagus 2022;5:17.




