
Medical management of acid/bile reflux before, during and after endoscopic therapy for Barrett’s esophagus: a narrative review
Introduction
It is well established that the main precursor to esophageal adenocarcinoma (EAC) is Barrett’s esophagus (BE) (1,2). Persistent gastric acid exposure to the distal esophagus has been identified in the pathogenesis of BE (2). The cascade of non-dysplastic Barrett’s to low grade dysplasia (LGD), high grade dysplasia (HGD) then cancer, provides an opportunity for endoscopic eradication therapy (EET) to not only halt the progress but also completely eradicate metaplastic and dysplastic epithelium (3,4). Successful eradication after EET is however limited in the presence of ongoing sources of inflammation such as reflux causing re-injury. Uncontrolled reflux has been identified as a risk factor for recurrence after EET. Esophageal pH optimization and acid reflux control pre-procedure, intra-procedure and post-procedure has been adopted as a key strategy in achieving good clinical outcomes (5,6). This review focuses on the critical importance of acid suppression optimization before, during and after EET. We present the following article in accordance with the Narrative Review reporting checklist (available at http://dx.doi.org/10.21037/aoe-20-74).
Background on BE and endotherapy
In the United States, BE is defined as a condition in which the normal stratified squamous epithelium of the distal esophagus is replaced by intestinal type epithelium with the presence of goblet cells (7). Histologically BE is reported without dysplasia, indefinite for dysplasia, LGD, HGD or intramucosal cancer (IMC) (8-10). The risk of progression to EAC in BE has been estimated at 0.2–0.5% per year with recent studies citing an even lower rate of progression of 0.18–0.3% per year in non-dysplastic BE, 0.5% per year in LGD and 7–15% per year in HGD (9-11).
EET is standard of care for those patients with HGD and IMC. EET also may be considered in select patients at LGD stage with a 25% reduction in progression to HGD/EAC when compared to surveillance alone (12). Histologic classification of the degree of dysplasia is considered the most important predictive marker for risk of progression. This histologic classification also guides the gastroenterologist on the aggressiveness of acid suppression; it is widely accepted and supported in the literature that daily proton pump inhibitors (PPIs) therapy is recommended in patients without dysplasia but twice a day is recommended when dysplasia is present and/or EET is considered (9,13). EET consists of tissue acquisition [endoscopic mucosal resection (EMR)], ablative therapy [radiofrequency ablation (RFA), cryotherapy, laser, multi-polar electrocoagulation, argon plasma coagulation, photodynamic therapy] or a combination of both (hybrid/multimodal therapy). RFA is the most frequently used and studied of the ablative therapies with proven durability. The goal of EET is complete eradication of intestinal metaplasia (CE-IM) by directly treating the neoplastic area and any at-risk areas of Barrett’s mucosa (14).
Inadequate acid suppression defined by impedance-pH monitoring is a modifiable risk factor and increases the risk of recurrence of intestinal metaplasia (IM) after EET. Ongoing distal esophageal reflux, both acid and non-acid is also associated with incomplete response to EET (15). This observation highlights the importance of aggressive pharmacologic acid suppression and also reminds us of the role of lifestyle modifications in decreasing distal esophageal reflux exposure both during and after the EET period.
The reflux-inflammation-Barrett’s cascade
Acid reflux
Up to one third of the American population has reported regular reflux symptoms but only 10% of these individuals progress to BE. It is also notable that not all patients with BE report symptoms of reflux, though the magnitude of the risk for BE and EAC has been reported to be higher in those with prolonged symptoms of gastroesophageal reflux and early age of reflux symptoms (16-18). Symptom control is also a poor predictor of acid control with pH normalization seen at best in 85% of individuals on PPIs (17).
Acid reflux has been shown to induce inflammatory changes in the distal esophagus through cyclo-oxygenase-2 (COX-2), c-myc and mitogen-activated protein kinase signaling (16,19,20). There has been ex-vivo observation of overexpression of COX-2 in EAC and metaplastic Barrett’s epithelium (19,20).
Barrett’s metaplasia not only develop but also proliferate in acidic conditions and this proliferation is aborted when pH normalization occurs (21). pH normalization does not occur in all patients treated with high dose PPIs and adjunctive measures should also be considered to decrease distal esophageal reflux exposure and improve the likelihood of successful EET.
Bile reflux
Bile reflux or duodenogastric reflux has been proposed in the etiology of BE as bile acids have been found in the refluxate of patients with BE. It has been suggested that bile acids may exert their effects on the esophageal mucosa through cytotoxic pathways and upregulation of proto-oncogene and c-myc resulting in inflammation and contributing to the inflammation-cancer cascade (16). Bile acids become non- ionized at acidic pH, enters cells and exert mucosal injury and inflammation (Figure 1) (9,17,22). The effect of bile acid has been found to be most significant when combined with gastric acid.
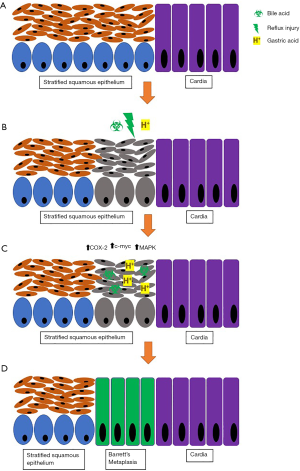
Inflammation
In vitro studies have demonstrated pro-proliferative and anti- apoptotic features when esophageal cells are exposed to acidic media. Similarly, ex vivo Barrett’s tissue has exhibited similar features with pulsed acid exposure. Acid suppression mitigates these findings through pH normalization and thus improves outcomes after EET (17,21).
The relative insensitive Barrett’s mucosa to reflux, make symptom control a poor variable for adequate reflux control and inflammation and symptoms alone should not be used as a predictor of pH normalization (15,17). It is the responsibility of the endoscopist to ensure medical management is optimized to ensure pH normalization. PPI therapy is standard of care in patients with BE with or without dysplasia and with or without EET (4,8,9).
The mechanism of action of PPI therapy
PPIs increase the intragastric pH by blocking the H+/K+ ATPase pump, the major site of gastric acid release in the stomach. There has been debate regarding the use of PPIs in preventing the neoplastic progression from BE to HGD or IMC (8). In 2008, however, a large retrospective study by Hillman et al. found that acid suppression with PPI therapy stabilizes cell proliferative activity in BE and long term PPI therapy decreases the rate of neoplastic progression of BE (23). PPI use has also been shown to facilitate the regression of metaplastic epithelium (21,23).
PPIs have not been shown to shorten the length of the Barrett’s segment (a risk factor for recurrence of IM after EET), though the data is still conflicting. It has however been shown that strictures, ulcers and nodularity found during endoscopic surveillance increase the risk of progression from non-dysplastic BE to neoplastic BE by a factor of 6.7. These lesions are often found in uncontrolled acid environments in the distal esophagus, and treatment often includes PPI therapy (23,24).
One meta- analysis reported a 71% risk reduction in progression to HGD/EAC (25) with PPI use. The benefit observed in most studies was duration dependent with most benefit observed after 6 months of continuous use but maximal benefit was seen after 2–3 years of continuous use (25,26). PPI use has been directly correlated with the formation of squamous islands/squamous re-epithelialization in mucosa adjacent to and above Barrett’s mucosa and leads to a decreased incidence of EAC (27). PPIs have a key role in improving the intra-esophageal pH environment and when combined with EET, has been shown to optimize squamous re-epithelialization (5).
The role of PPIs in bile acid suppression has been debated. There has been a notable decrease in bile reflux seen with PPI use but the degree of improvement is significantly less than that observed for acid reflux—50% vs. almost 80% reduction, respectively (16,28,29). The mechanism of action with PPI use in bile acid suppression is uncertain and some theories include decreased gastric volume, increased esophageal motility and gastric clearance but these mechanisms have yet to be proven in randomized controlled studies.
Factors associated with recurrence of IM after RFA
Some factors associated with recurrence of IM include long segment BE (>3 cm)—an independent risk factor also associated with increased risk of adenocarcinoma, advanced histology, increased number of treatment sessions to achieve CE-IM, poor reflux control or the presence of a hiatal hernia (15,30,31).
An observational study by Krishnan et al. noted that decreased durability, uncontrolled reflux before EET and incomplete response to high dose PPIs were associated with persistence of IM after RFA (15). The prospective study by Komanduri et al. looked at optimization of reflux control on recurrence of IM after EET with EMR and/or RFA. A key intervention was the use of a standardized reflux management protocol. This protocol involved: (I) counseling on the importance of PPI therapy and the importance of adherence to this medication pre-EET; (II) PPI therapy optimization with twice a day dosing and timed 30 min before meals; (III) reflux symptom monitoring and medication adherence at each visit during the BE surveillance period and (IV) an on-treatment 24 pH-impedance and high resolution manometry was performed on those with ongoing reflux symptoms, endoscopic evidence of esophagitis during EET or inability to achieve CE-IM after 3 RFA sessions. Patients who had an abnormal on-treatment 24 pH-impedance test were referred for a fundoplication. In this well-designed study, 221 participants with dysplastic BE or high risk non-dysplastic BE underwent EET and were compared with 64 historical controls. Using this structured reflux management protocol, 93% of participants achieved CE-IM and 96% achieved complete eradication of dysplasia. Interestingly, the only predictive factor for incomplete responders (n=64) who did not achieve CE-IM after 3 ablative sessions was a reduction in PPI dosing or frequency—either due to personal choice, insurance mandates or prescribing by another physician. Furthermore, of the incomplete responders who underwent pH-impedance testing (n=48), 81% required a fundoplication. When compared to historical controls, the authors concluded that although the rate of CE-IM was similar (93% vs. 88%, P=0.19), utilizing a structured reflux management protocol led to a significant decrease in the recurrence rate of IM (4.8% vs. 10.9%, P=0.04). This study strongly supports the importance of aggressive reflux management pre, during and post EET (13).
In patients with even weakly acidic refluxes, the association with recurrence after EET has been seen, solidifying the importance of high dose aggressive acid control peri- EET.
Reflux control requires both lifestyle modifications and pH control. Isolated PPI therapy alone may fail to normalize intra-esophageal pH and combination therapy may need to be considered to optimize response to EET. It has been demonstrated that decreasing the total number of non-acid refluxes may also improve outcomes in patients undergoing EET.
Suggested reflux modification strategies for patients with BE undergoing EET: (I) pharmacologic methods for pH optimization: high dose twice daily PPI therapy, appropriately timed before breakfast and dinner, and consider a histamine 2 blocker at bedtime; (II) adjunctive pharmacologic methods for symptom and reflux control: alginate products which have been shown to decrease post- prandial reflux by displacing the gastric acid pocket and (III) non-pharmacologic methods for symptom and reflux control involves volume control, avoiding trigger foods and nocturnal regurgitation. Common and proven recommendations to achieve this include head of bed elevation and laying on the left side, avoiding late meals and avoiding foods such as chocolates, citrus or acidic foods such as tomatoes, carbonated beverages, fatty or fried foods, coffee, tobacco use, alcohol use and weight loss (32-35). Acid and non-acid reflux control utilizing these strategies aim to optimize outcomes with EET in dysplastic and neoplastic BE.
Recommended reflux protocols for patients undergoing EET
While there is some variation from institution to institution, general recommendations for protocols peri-EET for Barrett’s associated neoplasia are listed in Table 1 and include twice daily PPI, dissolved or suspension sucralfate, a topical lidocaine mixture and a liquid diet for 1 -2 days followed by a soft diet for up to a week after EET. Analgesia may be provided with acetaminophen and/or other non NSAIDS products if needed.

Full table
Once CE-IM is achieved, aggressive control of reflux is recommended as the rate of recurrence of IM after EET has been estimated to be 5–10% per year (36-38).
It is worthwhile mentioning that studies demonstrating long term durability of RFA were performed in patients on high dose, twice daily PPI maintenance therapy (39).
Anti-reflux surgery may be considered for patients with inadequate control of reflux symptoms despite optimization of medical therapy.
Conclusions
Single modality or multimodal EET using EMR and ablative therapies have been the standard of care for treating BE with LGD, HGD or IMC. EET is most successful when conducted in conjunction with aggressive reflux control before, during and after therapy. Successful eradication, vigilant surveillance monitoring, and optimal antireflux control together are poised to ultimately lead to improved patient outcomes and decrease recurrence of dysplasia and IM.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Madhav Desai) for the series “Endoscopic Therapy for Barrett’s Esophagus” published in Annals of Esophagus. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/aoe-20-74
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoe-20-74). The series “Endoscopic Therapy for Barrett’s Esophagus” was commissioned by the editorial office without any funding or sponsorship. VJAK reports grants from Ironwood, grants from Pentax, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Spechler SJ. Barrett’s esophagus. N Engl J Med 2002;346:836-42. [Crossref] [PubMed]
- Souza RF. From reflux esophagitis to esophageal adenocarcinoma. Dig Dis 2016;34:483-90. [Crossref] [PubMed]
- Spechler SJ. Barrett’s esophagus Semin. Oncol 1994;21:431-7. [PubMed]
- Sharma P, Katzka DA, Gupta N, et al. Quality indicators for the management of Barrett’s esophagus, dysplasia, and esophageal adenocarcinoma: international consensus recommendations from the American Gastroenterological Association Symposium. Gastroenterology 2015;149:1599-606. [Crossref] [PubMed]
- Akiyama J, Marcus SN, Triadafilopoulos G. Effective intra-esophageal acid control is associated with improved radiofrequency ablation outcomes in Barrett’s esophagus. Dig Dis Sci 2012;57:2625-32. [Crossref] [PubMed]
- Wani S, Muthusamy VR, Shaheen NJ, et al. Development of quality indicators for endoscopic eradication therapies in Barrett’s esophagus: the TREAT-BE (Treatment with Resection and Endoscopic Ablation Techniques for Barrett’s Esophagus) Consortium. Gastrointest Endosc 2017;86:1-17. [Crossref] [PubMed]
- American Gastroenterological Association. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology 2011;140:1084-91. [Crossref] [PubMed]
- Stier MW, Konda VJ, Hart J, et al. Post-ablation surveillance in Barrett’s esophagus: A review of the literature. World J Gastroenterol 2016;22:4297. [Crossref] [PubMed]
- Shaheen NJ, Falk GW, Iyer PG, et al. ACG clinical guideline: diagnosis and management of Barrett’s esophagus. Am J Gastroenterol 2016;111:30-50. [Crossref] [PubMed]
- Bennett C, Moayyedi P, Corley DA, et al. BOB CAT: a large-scale review and Delphi consensus for management of Barrett’s esophagus with no dysplasia, indefinite for, or low-grade dysplasia. Am J Gastroenterol 2015;110:662. [Crossref] [PubMed]
- Wani S, Falk GW, Post J, et al. Risk factors for progression of low-grade dysplasia in patients with Barrett’s esophagus. Gastroenterology 2011;141:1179-86. [Crossref] [PubMed]
- Wani S, Sharma P. Challenges with endoscopic therapy for Barrett’s esophagus. Gastroenterol Clin North Am 2015;44:355-72. [Crossref] [PubMed]
- Komanduri S, Kahrilas PJ, Krishnan K, et al. Recurrence of Barrett’s esophagus is rare following endoscopic eradication therapy coupled with effective reflux control. Am J Gastroenterol 2017;112:556-66. [Crossref] [PubMed]
- Konda VJ, Waxman I. Endotherapy for Barrett’s esophagus. Am J Gastroenterol 2012;107:827-33. [Crossref] [PubMed]
- Krishnan K, Pandolfino JE, Kahrilas PJ, et al. Increased risk for persistent intestinal metaplasia in patients with Barrett’s esophagus and uncontrolled reflux exposure before radiofrequency ablation. Gastroenterology 2012;143:576-81. [Crossref] [PubMed]
- Raj A, Jankowski J. Acid suppression and chemoprevention in Barrett’s oesophagus. Dig Dis 2004;22:171-80. [Crossref] [PubMed]
- Jankowski JA, Anderson M. management of oesophageal adenocarcinoma—control of acid, bile and inflammation in intervention strategies for Barrett’s oesophagus. Aliment Pharmacol Ther 2004;20:71-80. [Crossref] [PubMed]
- Green JA, Amaro R, Barkin JS. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. Dig Dis Sci 2000;45:2367-8. [Crossref] [PubMed]
- Souza RF, Shewmake K, Beer DG, et al. Selective inhibition of cyclooxygenase-2 suppresses growth and induces apoptosis in human esophageal adenocarcinoma cells. Cancer Res 2000;60:5767-72. [PubMed]
- Souza RF, Shewmake K, Pearson S, et al. Acid increases proliferation via ERK and p38 MAPK-mediated increases in cyclooxygenase-2 in Barrett’s adenocarcinoma cells. Am J Physiol Gastrointest Liver Physiol 2004;287:G743-8. [Crossref] [PubMed]
- Leedham S, Jankowski J. The evidence base of proton pump inhibitor chemopreventative agents in Barrett’s esophagus—the good, the bad, and the flawed! Am J Gastroenterol 2007;102:21-3. [Crossref] [PubMed]
- Batzri S, Harmon JW, Schweitzer EJ, et al. Bile acid accumulation in gastric mucosal cells. Proc Soc Exp Biol Med 1991;197:393-9. [Crossref] [PubMed]
- Hillman LC, Chiragakis L, Shadbolt B, et al. Effect of proton pump inhibitors on markers of risk for high‐grade dysplasia and oesophageal cancer in Barrett’s oesophagus. Aliment Pharmacol Ther 2008;27:321-6. [Crossref] [PubMed]
- Cooper BT, Chapman W, Neumann CS, et al. Continuous treatment of Barrett’s oesophagus patients with proton pump inhibitors up to 13 years: observations on regression and cancer incidence. Aliment Pharmacol Ther 2006;23:727-33. [Crossref] [PubMed]
- Singh S, Garg SK, Singh PP, et al. Acid-suppressive medications and risk of oesophageal adenocarcinoma in patients with Barrett’s oesophagus: a systematic review and meta-analysis. Gut 2014;63:1229-37. [Crossref] [PubMed]
- Martinucci I, de Bortoli N, Russo S, et al. Barrett’s esophagus in 2016: From pathophysiology to treatment. World J Gastrointest Pharmacol Ther 2016;7:190. [Crossref] [PubMed]
- Amadi C, Gatenby P. Barrett’s oesophagus: Current controversies. World J Gastroenterol 2017;23:5051. [Crossref] [PubMed]
- Katz PO. Management of the patient with Barrett’s esophagus: a continuing dilemma for the clinician. Rev Gastroenterol Disord 2004;4:49-59. [PubMed]
- Kunsch S, Neesse A, Linhart T, et al. Impact of pantoprazole on duodeno-gastro-esophageal reflux (DGER). Z Gastroenterol 2009;47:277-82. [Crossref] [PubMed]
- Pasricha S, Bulsiewicz WJ, Hathorn KE, et al. Durability and predictors of successful radiofrequency ablation for Barrett’s esophagus. Clin Gastroenterol Hepatol 2014;12:1840-7. [Crossref] [PubMed]
- Menke‐Pluymers MB, Hop WC, Dees J, et al. Risk factors for the development of an adenocarcinoma in columnar‐lined (Barrett) esophagus. Cancer 1993;72:1155-8. [Crossref] [PubMed]
- Patti MG. An evidence-based approach to the treatment of gastroesophageal reflux disease. JAMA Surg 2016;151:73-8. [Crossref] [PubMed]
- Sethi S, Richter JE. Diet and gastroesophageal reflux disease: role in pathogenesis and management. Curr Opin Gastroenterol 2017;33:107-11. [Crossref] [PubMed]
- Leiman DA, Riff BP, Morgan S, et al. Alginate therapy is effective treatment for GERD symptoms: a systematic review and meta-analysis. Dis Esophagus 2017;30:1-9. [Crossref] [PubMed]
- Kroch DA, Madanick RD. Medical treatment of gastroesophageal reflux disease. World J Surg 2017;41:1678-84. [Crossref] [PubMed]
- Reed CC, Shaheen NJ. Management of Barrett Esophagus Following Radiofrequency Ablation. Gastroenterol Hepatol (N Y) 2019;15:377. [PubMed]
- Orman ES, Kim HP, Bulsiewicz WJ, et al. Intestinal metaplasia recurs infrequently in patients successfully treated for Barrett’s esophagus with radiofrequency ablation. Am J Gastroenterol 2013;108:187-95; quiz 196. [Crossref] [PubMed]
- Phoa KN, Pouw RE, Van Vilsteren FG, et al. Remission of Barrett’s esophagus with early neoplasia 5 years after radiofrequency ablation with endoscopic resection: a Netherlands cohort study. Gastroenterology 2013;145:96-104. [Crossref] [PubMed]
- Shaheen NJ, Overholt BF, Sampliner RE, et al. Durability of radiofrequency ablation in Barrett’s esophagus with dysplasia. Gastroenterology 2011;141:460-8. [Crossref] [PubMed]
Cite this article as: Jaswani T, Ellison AC, Konda VJA. Medical management of acid/bile reflux before, during and after endoscopic therapy for Barrett’s esophagus: a narrative review. Ann Esophagus 2021;4:13.


