
Esophageal cancer with signet-ring cell features is associated with poor prognosis in the modern treatment era: factors influencing overall and disease-free survival
Introduction
Adenocarcinoma is the most common histologic subtype of esophageal cancer (1) in the United States, and signet-ring cell carcinoma is a subtype of adenocarcinoma characterized by the abundant production of intracellular mucin, which displaces and compresses the nucleus to the periphery of the cell, thus creating the characteristic crescent or signet-ring shape (2). The standard of care for patients with locally advanced esophageal cancer is multimodality therapy, with surgery following neoadjuvant, platinum-based doublet chemotherapy and radiation (3).
Signet ring cell differentiation in esophageal adenocarcinoma, prior to the now-standard induction chemoradiation followed by surgery protocols, was associated with reduced overall and disease-free survival, less down-staging, significantly lower rates of complete pathologic response, and a higher rate of positive margins, when compared to those patients with adenocarcinoma without signet-ring cell features (4-9). Available literature regarding signet-ring cell esophageal cancer stems from surgical databases, which cannot provide insight into patients who ultimately fail induction therapy. The purpose of this study was to evaluate the of impact modern treatment strategies and to identify factors influencing overall and disease-free survival in esophageal cancer with signet-ring cell features. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/aoe-20-55).
Methods
All patients with the diagnosis of esophageal cancer with signet ring cell features, who were treated at our institution between 1996 and 2018 were included in this study. Patients were identified through a system-wide database. Patients were excluded if they ultimately received care outside this specific institution or if the disease was truly confined to the stomach (gastric cancer). Patient clinical, operative, and pathologic data was retrospectively reviewed. CROSS-style induction chemoradiation was widely adopted and applied by 2012, and this time period was chosen for the outcome analysis. Survival calculations were performed with standard Kaplan-Meier estimators. Categorical variables were compared using a Fisher’s exact test, and a Wilcoxon rank-sum test was used for continuous variables. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Partners Human Research Committee of Partners HealthCare (#2014P000998). Because of the retrospective nature of the research, the requirement for informed consent was waived.
Results
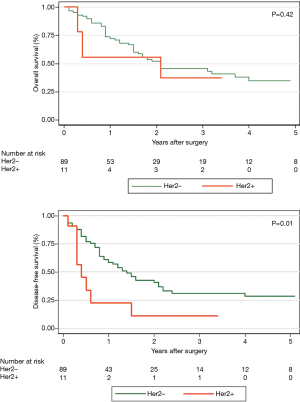
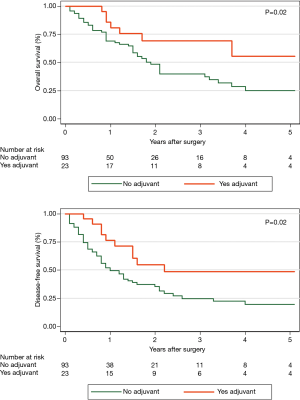
A total of 535 patients with esophageal cancer with signet-ring cell features were identified in the database, 72 (13.5%) of which were metastatic on presentation. Eighty-five (15.9%) had cancers confined to the stomach and were excluded. One hundred and eighteen patients were treated at different hospitals outside of the state or were treated at sister hospitals within our health system. This left 260 patients treated with curative intent at our institution. CROSS-style induction chemoradiation was widely adopted and applied by 2012, and this time period was chosen for the analysis. As such, this left 147 patients treated with curative intent for esophageal cancer with signet-ring cell features between 2012 and 2018 (Figure 1). Thirty-one patients were unresectable at the time of planned esophagectomy or progressed on re-staging imaging and did not reach esophagectomy (21.1%). This left 116 patients who underwent esophagectomy between 2012 and 2018. Mean age was 63 years. Ninety-seven patients had information about pre-treatment EUS. Of those 97 patients, 46 (47.4%) were down-staged, 35 (36.1%) were unchanged and 16 (16.5%) were upstaged on final pathologic review. R0 resection was achieved in 94.8% of patients (n=110). Pathologic complete response occurred in only 9.5% of patients. Five-year overall survival (OS) for patients with esophageal cancer with signet-ring cell features was 31.1%, and median disease-free survival (DFS) was 15 months, considerably less than historically reported for adenocarcinomas. HER2 testing was performed in 89.7% of surgical patients and 11 patients were HER2 positive (10.6%). Patient demographics and preoperative staging were not different between the two groups (Table 1). Hospital length of stay, overall complications, number of lymph nodes sampled, and length of stay did not differ according to HER2 status (Table 2). Due to the small sample size and relative rarity of events, there was a difference in pneumonia and reintubation, with HER2+ patients having proportionately higher rates (Table 2). Patients with HER2+ expression experienced a trend toward decreased overall survival, and none were alive at 5 years (compared to n=8, 34.8% in HER2− patients, P=0.388). HER2 positive expression conferred significantly worse disease-free survival (median DFS 5 vs. 17 months, P=0.016) (Figure 2). Twenty-one patients (18.1%) out of the entire cohort (n=116) received adjuvant chemotherapy, and 4 patients received adjuvant radiation. The overall and disease-free survival of patients who received adjuvant therapy were significantly better than that of those who did not receive adjuvant therapy (Figure 3). Among resectable cancers, recurrence was common (n=52, 44.8%) and the majority of recurrences were systemic (n=42, 80.8%).
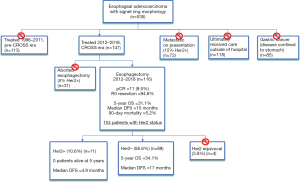
Table 1
| Characteristic | Total (n=100) | HER2+ (n=11) | HER2− (n=89) | P value |
|---|---|---|---|---|
| Age (years) | ||||
| Mean ± SD | 62.8±11.6 | 64.7±12.3 | 62.6±11.5 | 0.720 |
| Median (range) | 64 (26.4–84.9) | 68.3 (44–84.9) | 64 (26.4–82.5) | |
| Gender | ||||
| Male | 91 (91%) | 10 (90.9%) | 81 (91%) | 1.000 |
| Smoking status | ||||
| Never | 35 (35%) | 3 (27.3%) | 32 (36%) | 0.507 |
| Current | 43 (43%) | 4 (36.4%) | 39 (43.8%) | |
| Former | 22 (22%) | 4 (36.4%) | 18 (20.2%) | |
| Barrett’s esophagus | 23 (23%) | 1 (9.1%) | 22 (24.7%) | 0.449 |
| Atrial fibrillation | 6 (6%) | 0 (0%) | 6 (6.7%) | 1.000 |
| Other cancer | 16 (16%) | 3 (27.3%) | 13 (14.6%) | 0.376 |
| Congestive heart failure | 1 (1%) | 0 (0%) | 1 (1.1%) | 1.000 |
| Coronary artery disease | 15 (15%) | 3 (27.3%) | 12 (13.5%) | 0.363 |
| COPD | 7 (7%) | 1 (9.1%) | 6 (6.7%) | 0.570 |
| Hypertension | 54 (54%) | 5 (45.5%) | 49 (55.1%) | 0.750 |
| Diabetes mellitus | 16 (16%) | 1 (9.1%) | 15 (16.9%) | 1.000 |
| Clinical staging (c) | ||||
| No EUS done | 6 (6%) | 0 (0%) | 6 (6.7%) | 0.495 |
| Stage 1 | 2 (2%) | 0 (0%) | 2 (2.2%) | |
| Stage 2 | 16 (16%) | 0 (0%) | 16 (18%) | |
| Stage 3 | 68 (68%) | 10 (90.9%) | 58 (65.2%) | |
| Stage 4 | 8 (8%) | 1 (9.1%) | 7 (7.9%) | |
| Pathologic staging (yp) | ||||
| Stage 1 | 25 (25%) | 3 (27.3%) | 22 (24.7%) | 0.271 |
| Stage 2 | 20 (20%) | 2 (18.2%) | 18 (20.2%) | |
| Stage 3 | 44 (44%) | 3 (27.3%) | 41 (46.1%) | |
| Stage 4 | 11 (11%) | 3 (27.3%) | 8 (9%) | |
| Lymph nodes sampled | ||||
| Mean ± SD | 18±7.9 | 18.4±8.6 | 18±7.9 | 0.774 |
| Median (range) | 17 (0 to 41) | 21 (1 to 33) | 17 (0 to 41) | |
| Positive lymph nodes | ||||
| Mean ± SD | 2.4±4.1 | 3.5±5.4 | 2.2±3.9 | 0.894 |
| Median (range) | 1 (0 to 22) | 0 (0 to 16) | 1 (0 to 22) | |
| Surgical approach | ||||
| Minimally invasive | 73 (73%) | 7 (63.6%) | 66 (74.2%) | 0.661 |
| Open | 17 (17%) | 3 (27.3%) | 14 (15.7%) | |
| Hybrid | 10 (10%) | 1 (9.1%) | 9 (10.1%) | |
| Operation type | ||||
| Ivor Lewis | 62 (62%) | 8 (72.7%) | 54 (60.7%) | 0.839 |
| Three-Hole | 34 (34%) | 3 (27.3%) | 31 (34.8%) | |
| Other | 4 (4%) | 0 (0%) | 4 (4.5%) | |
| Median length of stay (days) | 9 | 9 | 9 | 0.815 |
SD, standard deviation; COPD, chronic obstructive pulmonary disease; EUS, endoscopic ultrasound.
Table 2
| Complication | Total (n=100), n [%] | HER2+ (n=11), n [%] | HER2− (n=89), n [%] | P value |
|---|---|---|---|---|
| Overall complications | 40 [40] | 5 [45.5] | 35 [39.3] | 0.751 |
| Pulmonary embolism | 1 [1] | 0 [0] | 1 [1.1] | 1.000 |
| Pleural effusion | 5 [5] | 0 [0] | 5 [5.6] | 1.000 |
| Reintubation | 6 [6] | 3 [27.3] | 3 [3.4] | 0.017 |
| Aspiration | 12 [12] | 2 [18.2] | 10 [11.2] | 0.618 |
| Pulmonary edema | 1 [1] | 0 [0] | 1 [1.1] | 1.000 |
| ARDS | 1 [1] | 0 [0] | 1 [1.1] | 1.000 |
| Pneumonia | 14 [14] | 4 [36.4] | 10 [11.2] | 0.045 |
| Empyema | 5 [5] | 0 [0] | 5 [5.6] | 1.000 |
| Wound Infection | 3 [3] | 1 [9.1] | 2 [2.2] | 0.298 |
| Chyle leak | 2 [2] | 0 [0] | 2 [2.2] | 1.000 |
|
|
1 [1] | 0 [0] | 1 [1.1] | 1.000 |
| Urinary tract infection | 1 [1] | 0 [0] | 1 [1.1] | 1.000 |
| Recurrent laryngeal nerve paresis | 4 [4] | 1 [9.1] | 3 [3.4] | 0.377 |
| Acute kidney injury | 3 [3] | 1 [9.1] | 2 [2.2] | 0.298 |
| Take back to OR | 17 [17] | 3 [27.3] | 14 [15.7] | 0.392 |
| Anastomotic leak | 13 [13] | 1 [9.1] | 12 [13.5] | 1.000 |
| Delayed conduit emptying | 8 [8] | 0 [0] | 8 [9] | 0.593 |
| Hiatal hernia | 1 [1] | 0 [0] | 1 [1.1] | 1.000 |
| Readmittance | 16 [16] | 1 [9.1] | 15 [16.9] | 1.000 |
| Perioperative death | 2 [2] | 0 [0] | 2 [2.2] | 1.000 |
ARDS, acute respiratory distress syndrome.
Discussion
Signet-ring cell is traditionally thought of as a rare subset of patients with esophageal cancer. As such, randomized and non-randomized clinical trials have heretofore included these patients within the broader context of adenocarcinoma. Esophageal cancer patients with signet-ring cell features are thus treated with the standard of care trimodality therapy with little consideration to this this histologic classification that has shown to portend fewer pathologic complete responses and reduced overall and disease-free survival (8,9). Patel et al. reviewed esophageal signet-ring cell cancer patients treated with chemoradiation between 2000 and 2012,compared them to a reference group that excluded signet-ring cell cancers, and found that patients in the signet-ring cell group had a lower rate of complete pathologic response (9% vs. 26%, P<0.001) and more frequent positive margins (8). Yendamuri et al. (9) reviewed the SEER database between 2000 and 2004 and identified 596 patients with signet-ring cell; also finding worse overall survival both in surgical and non-surgical patients compared to non-signet-ring cell patients. Additionally, 42.3% of the signet-ring cell study group of patients were under 65, and the median age of patients in our study was 63, suggesting that this worrisome biology is affecting relatively young patients.
We found that 20% of patients treated with curative intent will fail induction chemoradiation-which highlights that the success rate of traditional multimodality therapy leaves significant opportunity for improvement. Chirieac et al. (6) reviewed esophageal adenocarcinoma specimens in the context of surgery alone vs. chemoradiation and found that signet-ring cell histology made up 17% of esophagectomy specimens. They found survival was significantly increased in patients who underwent induction chemoradiation vs. surgery alone and concluded that acellular mucin (but no viable carcinoma) was a positive pathologic sign following induction therapy.
The much-celebrated CROSS trial changed the standard of care for all esophageal cancer patients by increasing overall survival from 34% (with surgery alone) to 47% (with chemoradiation plus surgery) at 5 years. There was also a concomitant increase in adenocarcinoma-specific median disease-free survival from 17.7 to 29.9 months. Comparatively, in our study, esophageal cancer with signet-ring cell features, even with chemoradiation followed by surgery, afforded a 5-year overall survival of 31.1% and a median disease-free survival of just 15 months. Recognizing the limitations of our retrospective review, our best standard of care is just barely getting patients with this histology to where we were before the CROSS trial (10). There is currently no standard of care for the addition of adjuvant therapy to traditional chemoradiation followed by surgery strategies in esophageal cancer. Our study found a statistically significant improvement in overall (55.1% vs. 24.0%, P=0.02) and disease-free survival (48.2% vs. 19.3%, P=0.02) in esophageal cancer patients with signet-ring cell features who received additional adjuvant therapy. The combination of rather aggressive clinical behavior and relative resistance to established treatment strategies suggests patients with adenocarcinomas with signet-ring cell features of the esophagus and GE junction may benefit from a distinct, and particularly robust, treatment strategy. Additionally, operating room utilization comes at considerable cost—both literally for hospital and health systems as well as emotionally—for the patients preparing for esophagectomy after chemoradiation. Improving our ability to detect occult metastatic disease prior to planned esophagectomy would help mitigate the downstream costs of aborted esophagectomy in patients with esophageal cancer with signet-ring cell features. Research is emerging on the use of MRI as part of the re-staging process following chemoradiotherapy in patients with esophageal cancer and may have a unique role to play in this subset of patients as well (11-13).
Interest in targeted therapy for metastatic and unresectable gastric cancer ignited interest for such therapy in esophageal cancer as well, and much of that combined data has been extrapolated to pure esophageal cancer. The well-received ToGa trial (14) demonstrated improved survival with the addition of trastuzumab to standard chemotherapy regimens in metastatic and unresectable patients, though gastroesophageal junction cancers made up only a fraction of the patient population. It had previously been unknown what percentage of esophageal adenocarcinoma patients with signet-ring cell features over-express HER2. Our study found that 10.6% of surgical patients had cancers that overexpressed HER2 and that up to 15% of patients who were metastatic on presentation had HER2 overexpression. Disease-free survival was significantly higher in HER2− patients. This finding was despite similar preoperative stage distribution, demographics, perioperative complications, and perioperative mortality rates among the two groups. Importantly though, the power of the statistical analysis is limited by small sample size. Whether trastuzumab pans out as a viable addition to induction chemoradiation strategies, as studied in RTOG 1010 (15), understanding both how the molecular footprint of adenocarcinoma with signet-ring cell features may differ from standard adenocarcinoma as well as and the interplay of HER2 expression will be important in developing and evaluating alternative treatment strategies in esophageal cancer.
Conclusions
Signet-ring cell features are present in up to 19% of patients undergoing surgery for esophageal cancer, and up to 21% of patients with this histology will fail CROSS-regimen induction chemoradiation. Patients with esophageal cancer with signet-ring cell features experience fewer pathologic complete responses and worse disease-free and overall survival despite modern induction therapy and surgery strategies. HER2 expression appears to portend a particularly poor prognosis. Recurrence is common is patients with esophageal cancer with signet-ring cell features, and a treatment strategy that includes additional adjuvant therapy experience improved overall and disease- free survival. More studies are needed to isolate and study esophageal cancer with signet-ring cell features as a unique subset of esophageal cancer. Treatment protocols are needed that lead to improved systemic control, enhanced resectability, and more sensitive re-staging tools in patients with esophageal cancer with signet-ring cell features.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/aoe-20-55
Data Sharing Statement: Available at http://dx.doi.org/10.21037/aoe-20-55
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoe-20-55). EM reports personal fees from The VeraMedica Institute LLC, outside the submitted work. RB reports grants from Medgenome, grants from Roche, grants from Verastem, grants from Merck, grants from Gristone, grants from Epizyme, grants from Siemens, grants from Celsisus, grants from NCI, grants from DoD, grants from NIH, outside the submitted work; in addition, RB has a patent 7,622,260 licensed to BWH, a patent 8,450,057 licensed to BWH, a patent 8,551,700 licensed to BWH, and a patent 9,446,050 licensed to BWH and Patents/Equity in Navigation Sciences. JOW reports personal fees from Ethicon and personal fees from Medtronic, outside the submitted work. SJW is a consultant for Covidien and Ethicon, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Partners Human Research Committee of Partners HealthCare (#2014P000998). Because of the retrospective nature of the research, the requirement for informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Esophageal cancer statistics. American Cancer Society website. Available online: https://www.cancer.org/cancer/esophagus-cancer.html
- Sung CO, Seo JW, Kim KM, et al. Clinical significance of signet-ring cells in colorectal mucinous adenocarcinoma. Mod Pathol 2008;21:1533-41. [Crossref] [PubMed]
- Little AG, Lerut AE, Harpole DH, et al. The Society of Thoracic Surgeons Practice Guidelines on the Role of Multimodality Treatment for Cancer of the Esophagus and Gastroesophageal Junction. Ann Thorac Surg 2014;98:1880-5. [Crossref] [PubMed]
- Pernot S, Voron T, Perkins G, et al. Signet-ring cell carcinoma of the stomach: Impact on prognosis and specific therapeutic challenge. World J Gastroenterol 2015;21:11428-38. [Crossref] [PubMed]
- Enlow JM, Denlinger CE, Stroud MR, et al. Adenocarcinoma of the esophagus with signet ring cell features portends a poor prognosis. Ann Thorac Surg 2013;96:1927-32. [Crossref] [PubMed]
- Chirieac LR, Swisher SG, Correa AM, et al. Signet-ring cell or mucinous histology after preoperative chemoradiation and survival in patients with esophageal or esophagogastric junction adenocarcinoma. Clin Cancer Res 2005;11:2229-36. [Crossref] [PubMed]
- Yoon HH, Khan M, Shi Q, et al. The prognostic value of clinical and pathologic factors in esophageal adenocarcinoma: a mayo cohort of 796 patients with extended follow-up after surgical resection. Mayo Clin Proc 2010;85:1080-9. [Crossref] [PubMed]
- Patel VR, Hofstetter WL, Correa AM, et al. Signet ring cells in esophageal adenocarcinoma predict poor response to preoperative chemoradiation. Ann Thorac Surg 2014;98:1064-71. [Crossref] [PubMed]
- Yendamuri S, Huang M, Malhotra U, et al. Prognostic implications of signet ring cell histology in esophageal adenocarcinoma. Cancer 2013;119:3156-61. [Crossref] [PubMed]
- Shapiro J, van Lanschot JJB, Hulshof MCCM, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. [Crossref] [PubMed]
- van Rossum PS, van Lier AL, van Vulpen M, et al. Diffusion-weighted magnetic resonance imaging for the prediction of pathologic response to neoadjuvant chemoradiotherapy in esophageal cancer. Radiother Oncol 2015;115:163-70. [Crossref] [PubMed]
- Fang P, Musall BC, Son JB, et al. Multimodal Imaging of Pathologic Response to Chemoradiation in Esophageal Cancer. Int J Radiat Oncol Biol Phys 2018;102:996-1001. [Crossref] [PubMed]
- Wang Z, Guo J, Qin J, et al. Accuracy of 3-T MRI for Preoperative T Staging of Esophageal Cancer After Neoadjuvant Chemotherapy, With Histopathologic Correlation. AJR Am J Roentgenol 2019;212:788-95. [Crossref] [PubMed]
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [Crossref] [PubMed]
- Safron H, principal investigator. RTOG 1010. Available online: https://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=1010
Cite this article as: Lee DN, Mazzola E, Bhagat R, Kucukak S, Bravo-Iñiguez CE, Bueno R, Patil DT, Mamon HJ, Jaklitsch M, Wee JO, Swanson SJ, White A. Esophageal cancer with signet-ring cell features is associated with poor prognosis in the modern treatment era: factors influencing overall and disease-free survival. Ann Esophagus 2020;3:32.


