
The impact of prehabilitation on surgical outcomes
Introduction
For esophageal as well as most solid cancers, the current established standard treatment includes surgery and concurrent medical therapies, both of which have been shown to significantly enhance prognosis (1,2). Despite improved effectiveness and wider indications, they impose a large physiological stress, and have detrimental effects on acute and long-term function, negatively impact health trajectories (3-5). Management of esophageal cancer in particular is associated with a high risk of malnutrition, postoperative complications, persistent deconditioning and muscle wasting, which can contribute to emotional distress and reduced quality of life (6,7). Due to the increased susceptibility of these patients to treatment-induced morbidity, the importance for multidisciplinary supportive preoperative interventions to counter the decline in physiological and functional reserves cannot be overstated. Specifically, physical, nutritional and mental status can influence surgical outcomes, functional recovery, adherence to antitumoral therapies, access to surgery and quality of life throughout the course of the disease. The current article will address the principal determinants of esophageal surgery outcomes, with a particular emphasis on intraoperative care (enhanced recovery after surgery, ERAS) and the preoperative behavioural interventions that characterize multimodal prehabilitation.
ERAS
ERAS was initially designed in early 2000 with a view towards reducing perioperative complications and hospital stay (8), and it rapidly evolved into a multidisciplinary approach incorporating evidence-based interventions throughout the entire perioperative period. It became clear that modulating the complexity of the physiological stress response could not be sufficiently achieved solely through the introduction of minimally invasive surgery or improved anesthetic techniques. A collaborative approach was needed to overcome the multiple obstacles that prolong recovery, such as pain, ileus, immobilization, starvation, fluid overload, thrombosis, and postoperative catabolism. In doing so, surgeons, anesthesiologists and nurses have ceased delivering care from their individual silos, transitioning to a broader integrative approach that improves the quality of care and empowers patients and caregivers.
The ERAS society recently published recommendations for improved perioperative management for esophagectomy (9); Table 1 summarizes the main points. It emphasizes the importance of surgical considerations (procedure, access and conduit), optimization of nutrition (pre- and post-operative), multimodal analgesic approaches, early tube removal, early progressive mobilization and routine respiratory physiotherapy (10). Several institutions have implemented elements of ERAS for esophagectomies and have reported improvements in length of hospital stay, costs, and postoperative pulmonary complications, suggesting a reduced burden imposed on patients and the healthcare system (10-12).

Full table
Despite evident improvements achieved with ERAS, a significant proportion of patients still experience complications, and, even in absence of significant morbidity, full recovery can take months. Evidently a wider approach is still needed. In addition to in-hospital care, many other interventions have the potential to impact surgical outcomes, if proactively implemented in the preoperative period (13). The main components of functional capacity, such as poor physical status, malnutrition and sarcopenia, and mental distress, are critical determinants of surgical outcomes (14). With this complexity in mind, prehabilitation aims to promote a coordinated, multidisciplinary preoperative care plan to prevent functional decline related to treatment and its subsequent consequences (14). The prehabilitation program adopted by our institution was built in continuity with enhanced recovery pathways and has overlapping features (see “preoperative care” in Table 1).
Predictably, the ERAS Society recently endorsed the role of prehabilitation in the perioperative care of esophagectomy, acknowledging the pivotal importance for a faster return to an acceptable level of function after surgery (9). Nonetheless, published in late 2018, the level of recommendation was weak since limited evidence was available for prehabilitation in upper GI surgery. However, since then this scenario has further evolved. The following illustrates the rationale underlying the individual elements of prehabilitation, and recent findings are narratively summarized.
Prehabilitation for esophageal cancer
The importance of postoperative rehabilitation on physical performance and recovery is well-recognized (15,16). However, the preoperative period constitutes a unique opportunity to address comorbidities and modifiable risk factors, improve functional capacity and address deficiencies in physiologic reserve, which might otherwise preclude surgery or significantly impede recovery (13,17). Poor nutritional status is a significant concern throughout the care continuum of esophageal adenocarcinoma. Disease symptomatology leads to poor oral intake with resultant negative protein and energy balances, which often results in malnutrition. Not surprisingly, there is a high incidence of unintended weight loss (>70%) and sarcopenia (26–75%) at diagnosis, which can worsen due to anti-cancer therapies and persist throughout the preoperative period (6,18). Anemia is prevalent in addition to several micro-nutrient deficiencies, and can further impair a patient’s health and functional status (19,20). If nutritional deficits proceed unchecked throughout the preoperative period they can result in deleterious effects on body composition, physiological reserves, short and long-term functional status and quality of life (20).
Moreover, for these patients, disease-related impairments are not the only concern. Surgery represents a significant physiologic stress necessitating increased energetic and nutritional requirements to facilitate healing (18,21). Loss of muscle mass is a significant consideration in the management of esophageal adenocarcinoma, because of the resultant deconditioning, which in turn increases the risk for dose-limiting toxicities and surgical morbidity (6,19,22). In addition, the metabolic stress of neoadjuvant therapy (NAT) has been well documented to often cause muscle wasting, physical deconditioning and a reduced functional capacity, which result in low tolerance to physical stressors (5,6). It has been reported that up to 70% of these patients are unable to complete their prescribed perioperative regimens due to dose-limiting toxicities (5,6,23). In 2014, Jack et al. investigated the prognostic power of fitness parameters on tolerance to NAT and survival. Following NAT, patients experienced a significant decline in exercise tolerance, measured with oxygen consumption (VO2) at anaerobic threshold (2.19 mL/kg/min, 95% CI, 1.47 to 2.91) and VO2 at peak (2.51, 95% CI, 1.55 to 3.47), in FEV1 and FVC, and hemoglobin (5). Lower baseline aerobic fitness was also adversely associated with completion of NAT, and 1-year survival. Similar declines in cardiopulmonary fitness, lung function and hemoglobin following NAT have been reported by Sinclair and colleagues (3). Anemia and iron deficiency can be further impaired after gastric resection. These findings emphasize the role of physical fitness as critical determinant of cancer care.
The disease and therapies affect different aspects of health and well-being. Consequently, the importance for frequent screening of patient psychological status cannot be overstated. It is unsurprising that disease-related symptoms, functional decline and poor prognosis frequently result in manifestations of anxiety and depression following diagnosis, which are often exacerbated by the side-effects of neoadjuvant therapies (7). It is important to address them, as they can have adverse effects on mental health, self-efficacy, compliance to clinical interventions, sleep patterns, fatigue and quality of life (7). High levels of anxiety and distress can also negatively affect the postoperative period increasing perception of pain, length of stay and prolong the rate of recovery (24).
Given the peculiar complexity of esophageal care management, we tend to define and address the functional status of these patients going beyond the classical identification of comorbidities, taking into account the multiple components that determines the physiological resilience to stressors (Figure 1) (25).
Nutrition
Malnutrition, commonly seen in gastrointestinal cancers, is associated with increased morbidity and mortality, longer length of hospital stay, reduction of treatment efficacy and increased toxicity (21,26-29). It may also worsen with disease progression and neoadjuvant therapies. Despite being an integral component of most esophageal cancer treatment, NAT regimens are not without significant systemic side-effects. Particularly with chemotherapy, patients can experience both relief of dysphagia and adverse symptoms such as nausea, stomatitis, diarrhea and vomiting, contributing to a further decline in nutritional status (19). Esophagectomy is also associated with a significant morbidity, which is exacerbated by preoperative malnutrition and low functional status. Postoperatively, protein and energy requirements are uniformly elevated, and often unmet given iatrogenic limitations to macronutrients. Compared to other oncologic surgeries, esophagectomy reported the highest rate of malnutrition even after surgical treatment, strongly associated with postoperative complications. After gastrectomy, up to 1 year can be necessary to recover to a normal nutritional status (30).
Systematic screening of nutritional status should be performed at the first visit, and repeated regularly at short-interval. Several validated tools are available, such as Nutritional Risk Screening 2002 (NRS 2002) and Scored Patient-Generated Subjective Global Assessment (PG-SGA) and its abridged version (31,32). Low BMI (18.5 kg/m2), unintentional weight loss (>10% or >5% over 3 months), low albumin, and nutritional symptoms are common risk factors easy to check. Focusing only on underweight and malnutrition is a common misconception, as obesity is not uncommon in patients with esophageal adenocarcinoma. Furthermore, loss of skeletal muscle mass is a main trait of sarcopenia and can happen with minimal changes in fat mass. In a recent study, sarcopenic obesity was present in 14% of patients with esophagogastric cancer (33). This is important to consider, given that sarcopenia may nor be overtly evident and is independently associated with poor survival and dose-limiting NAT toxicities (34,35). For all these reasons, and in spite of delaying surgery, nutritional therapy should be provided for at least two weeks if severe nutritional risks are detected (36), and should follow standardized approach (21).
Dietary counselling should be proposed to all patients at moderate-to-high nutritional risk undergoing any cancer treatments. When possible, oral nutrition and supplementation are preferable, and nutritional counselling is mandatory to guide the patient to consume smaller portion sizes, while increasing meal frequency and chewing, and often adopting modified texture diet (18). The use of artificial nutrition with oral supplementation of high-density protein and caloric beverages as well as liquid meal replacements is often utilized as a means to enhance nutritional support (21). Given the hypermetabolic and catabolic drive associated with the disease and its treatments, it is imperative for elevated energetic and protein requirements to be addressed throughout the perioperative period. ESPEN guidelines recommend that caloric intake be increased to 25–30 kcal/kg/day during this time. To the same extent, it is strongly recommended to increase consumption of high grade protein, up to 1.5–2.0 g/kg/day and, ideally, at least 25 grams at every meal (37). Moreover, exercise should also be considered to counter further protein catabolism, support anabolic processes, and reduce risks of sarcopenia (21,38,39). These goals can be particularly challenging in upper GI cancers. In case of insufficient dietary intake, enteral nutrition is recommended and preferred over parental route in an intact gastro-intestinal tract (40). If necessary, enteral feeding approaches may include gastrostomy, percutaneous radiologic gastrostomy or percutaneous endoscopic jejunostomy; however, the selected approach should be adapted to each patient’s clinical status and preferences.
The benefits of enteral feeding remain uncertain, and its routine use is not recommended by ERAS society (9). Rather, preoperative immune-enhancing diets seems to have a compelling rationale in gastro-esophageal cancer. By the reduction of the inflammatory response and oxidative stress induced by cancer and its various therapies, it can be an important element within a multimodal approach to treat cachexia (which, by definition, is a condition that cannot be fully reversed by conventional nutrition) (41,42). Several nutrients have been tested, such as omega-3 polyunsaturated fatty acids (PUFA), select amino acids (arginine and glutamine), nucleic acids, and several antioxidants (21). Omega-3 PUFA have unique anti-inflammatory properties and have been previously used in other oncologic populations (18). In advanced-stage cancers, it has been demonstrated promising potential to reduce basal metabolic rates, and reduce the inflammatory biomarkers and acute phase proteins. Additionally, it has been proposed to improve appetite, weight management and preservation of lean body mass in some advanced stage cancers (21).
Physical status
For years, bedrest was the main and unique approach to physical and mental fatigue associated with cancer. Fortunately, over the last 3 decades, exercise has established its role in attenuating and even reversing the adverse effects of cancer and its treatments on physical fitness, physical functioning, cancer-related fatigue, and quality of life (43). Benefits may vary according to the type of exercise, with aerobic and resistance training considered to be fundamental elements of most programs (44). This is particularly true for esophageal cancer due to their beneficial synergistic effects on functional status, capacity and quality of life (22). Across oncologic care, exercise has been reported to provide important impacts on disease progression, treatment efficacy and safety, and secondary prevention (45). From a personal perspective, exercise improves perceived physical status, mental health, and overall quality of life (46).
Cancer therapies adversely impact cardiorespiratory function and result in a predictable and progressive decline in aerobic fitness that persists even after treatment termination and negatively affects later functional status and quality of life (5). Fortunately, aerobic exercise offers several important physiological adaptations that can mitigate treatment-induced physiological and functional decline. It involves elevating heart rate through repetitive dynamic movements and can be performed with various modalities and can run the spectrum from continuous steady state to high-intensity interval training. In patients with cancer, aerobic training is well-recognized to increase maximal oxygen uptake, cardiac output, mitochondrial density, oxidative potential and peak power output (47). The combined result may lead to greater physiological reserves.
Resistance training can counter myopenia and promote hypertrophic adaptations in skeletal tissue, increasing muscle mass, strength and function. It has been shown to improve body composition, weight management and physical fitness in all age groups, but more importantly, in the frail and elderly (13,39,48). This can be pertinent in this population, as following NAT patients report an average loss of ~5 kg lean body mass, and ~4 kg in grip strength (49), indicative of a sharp increase in sarcopenic status, an independent predictor of postoperative complication risk and poor long‐term prognosis (50).
Psychosocial condition
The preoperative period of any major elective surgery is known to be associated with a high degree of distress, anxiety and depression (7). In esophageal adenocarcinoma, this commonly compounded by the poor prognosis and devastating physiological manifestations of the disease and contributes to poor treatment compliance and postoperative outcomes (51). In addition to specifically increasing pain perception, reducing functional capacity and HRQoL, psychological distress status has also been shown to reduce circulating immunological mediators, alter physiological mechanisms of wound healing, and increase length of stay and, as a result, augment healthcare costs (17,52,53).
Accordingly, it has become clear that prehabilitation programs should not only focus not only on improving physical health, but also psychosocial wellbeing with interventions geared towards reducing anxiety, depression, but also to promote patient engagement and empowerment (53). Although currently there is no consensus on what constitutes the optimal psychological prehabilitation program, most interventions generally involve private meetings with a psychologist or qualified healthcare professional who commonly utilize image-guided relaxation, stress reduction techniques, in addition to addressing problem solving and coping strategies (51,53).
State of evidence
Preoperative nutrition
Table 2 synopsizes the current evidence and controversies in nutrition therapy prior to esophago-gastric surgery. A Cochrane meta-analysis by Burden et al. demonstrated a significant reduction in postoperative complications when parental nutrition was provided prior to gastrointestinal surgery, but found no difference associated with standard oral supplementation or enteral nutrition (54). Research interests have also focused on immunonutrition and enteral supplementation of arginine, omega-3-fatty acids and ribonucleotides. In the same meta-analysis, seven trials focused on immunonutrition, showing a significant effect on improving postoperative morbidity (RR 0.67, 95% CI, 0.53 to 0.84) (54). In 2016, Wong et al. published a meta-analysis including 2016 patients who underwent esophagectomy, gastrectomy, and pancreatectomy; compared to standard enteral nutrition, immunonutrition lowered risk of infection, and shortened the length of hospital stay. However, entirely different conclusions can be drawn from other studies, which failed to show any clinical benefit of immune enhancing diets (55). Fujitani et al. found no difference in the incidence of infectious complications and overall morbidity in well-nourished patients undergoing elective gastrectomy (56); similar results were reported by Sultan et al., investing omega-3 PUFA supplementation in esophago-gastric surgery (57). No effect on postoperative complications and length of hospital stay was reported in two meta-analysis, that included 785 patients undergoing gastric surgery (58), and 628 patients undergoing esophago-gastric surgery (59). Given these unsettled indications, ERAS society does not support routine use of immunonutrition.
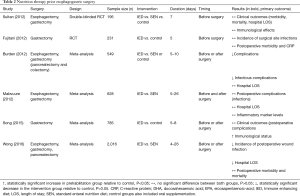
Full table
Physical training
Table 3 summarizes recent prospective trials exploring the effect of preoperative physical training before esophago-gastric surgery. The type of conditioning programs varies considerably between studies, specifically with respect to modality, supervision, duration, and outcome measures. Inspiratory muscle training (IMT) is one of the most investigated intervention, understood as a breathing exercise program aimed to improve the strength and the endurance of the respiratory muscles. Although data suggests that preoperative IMT can improve pulmonary function, the impact on postoperative pulmonary complications is unclear in patients undergoing esophagectomy. Inoue et al., conducted a retrospective analysis of 100 esophageal cancer patients, showing that “preoperative multimodal pulmonary rehabilitation” was associated with significant risk reduction for pulmonary complications (OR 0.14, 95% CI, 0.02 to 0.064) (68). Conversely, other prospective studies did not show any significant change in postoperative outcomes, such as functional walking capacity and pulmonary complications (60,66,69). In one of these trials, Valkenet et al. showed that a home-based high-intensity IMT programme did not reduce postoperative pneumonia compared with standard care (69). Several elements can possibly account for these results, such as the heterogenous duration of the intervention, the unsupervised approach and the relative low compliance to the protocol (only 54% of patients completed ≥80% of the prescribed training sessions and only 40% of all sessions were completed at the prescribed intensity). Nonetheless, this negative result is still surprising given the positive effect of IMT on preoperative pulmonary function (66,69).
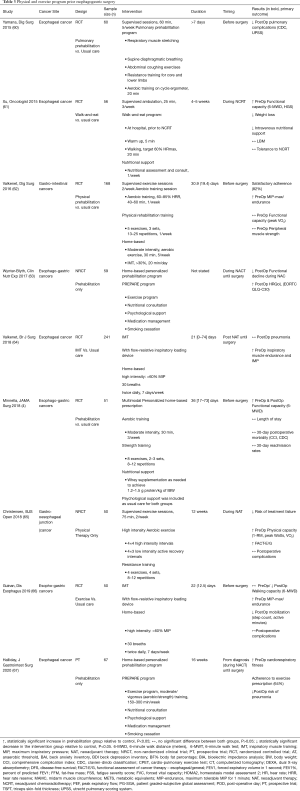
Full table
Christensen et al. recently investigated the potential role of physical prehabilitation in treatment tolerability and demonstrated that supervised exercise during NAT reduced the risk of failure to reach surgery (65). Serious adverse events that prevented surgical resection, such as disease progression or physical deterioration, occurred in 5% of patients in exercise groups vs. 21% in control (RR 0.23, 95% CI, 0.04 to 1.29). Main limitations of study were the relatively small sample size and the non-randomized design. Patients in the intervention group reported also a significant increase in functional status (mean FACT-E score: 9.6, 95% CI, 1.0 to 18.1), peak power output (12 Watts, 95% CI, 0.1 to 24.0), and peak VO2 (1.39 mL/kg/min, 95% CI, 0.03 to 2.74). Interestingly, although a continuous decline in weight was observed throughout the preoperative period, the exercise group did not experience significant changes in weight or lean body mass.
Barberan-Garcia et al. conducted an RCT involving 125 surgical candidates for major abdominal surgeries, which investigated the effect of prehabilitation on postoperative complications WR (70). The intervention group had supervised exercise sessions, 1–3 times per week and consisted of high-intensity interval training on a cycle ergometer with an intensity alternating between 40% and 70–85% of a patient’s baseline maximum work rate. Significant improvements in preoperative aerobic capacity were noted (endurance time 135%; P<0.001) in addition to a 51% reduction in the incidence of postoperative complications, as compared to controls (RR 0.5; 95% CI, 0.3–0.8, P=0.001). Unfortunately, only 18 participants (10 in prehabilitation and 8 in control group) underwent either esophageal or gastric surgery, and as such, the evidence in this population remains weak. A recent single-group, single-centre, prospective trial by Halliday et al. highlighted the importance exercise progress and volume in the context of multimodal prehabilitation junction (67). Beyond confirming a positive effect on preoperative physical fitness, the study showed that higher exercise volume was associated with lower pulmonary complication following curative esophageal resection.
Psychosocial intervention
Although the benefits of preoperative stress management have been proven to be reduce anxiety and depression in a number of oncologic populations, few studies have investigated the impact in esophageal cancer patients awaiting surgery (51). One study conducted Zhang et al., investigated the impact of a perioperative psychological support program in patients with carcinomas of the esophagus (71). The study utilized a multidisciplinary three-phase approach that included pre- and post-operative interventions with the aim of improving psychological wellbeing and postoperative outcomes in recently admitted surgical candidates (72). Psychological support throughout the perioperative period was reported to significantly improve postoperative multivariate measures of psychosomatic status but also the length of stay [20.06 (3.73) vs. 23.24 (7.37); P=0.041] in these patients. A similar study by Scarpa et al., investigated the impact of psychological support and sleep management strategies on HRQoL and self-reported sleep quality. The authors found that in comparison to usual care, the intervention group had less of a depreciation in HRQoL (OR: 0.23; 95% CI, 0.06 to 0.61) and sleep quality (OR: 0.27; 95% CI, 0.1 to 0.73) (72).
Multimodal strategies
In 2002, Persson et al. tested a multimodal approach in patients with gastrointestinal cancer. Using a 2×2 RCT design, they investigated the effect of nutritional, physical, and psychological support on physical condition and survival (73). A multimodal intervention resulted in a mild benefit in weight gain at 12 and 24 months, with no other differences detected. However, this study had several limitations: notably a small sample size of only 32 patients with gastric cancer, very low adherence to the nutrition plan (only half of the population reached 75% of the energy intake recommended), and no-surgical setting.
An RCT conducted by our group investigated the effect of multimodal prehabilitation on the changes in perioperative functional capacity, in surgical candidates with esophogastric cancers. In the RCT, 51 patients were recruited and randomized at diagnosis to either a control or prehabilitation group; the latter included a personalized dietary program in addition to a home-based exercise prescription provided by qualified healthcare professionals. Psychological interventions were offered to high-risk patients and included in the standard of care. All patients completed multidisciplinary assessments at baseline, preoperatively and 4–8 weeks postoperatively. The study revealed that prehabilitation resulted in significant improvements from with reference to baseline functional capacity preoperatively [change in 6-minute walk distance 36.9 (51.4) vs. ‒22.8 (52.5) m, P<0.001] and postoperatively [15.4 (65.6) vs. ‒81.8 (87.0) m, P<0.001] with respect to the control group, but with no significant changes in surgical and postoperative complications (4). Similarly, Wynter-Blyth and colleagues carried out an observational study investigating the impact of a multimodal prehabilitation program “PREPARE” in patients with esophago-gastric cancers scheduled to receive NACT and surgery. Although the patients did not experience an improvement in functional capacity as observed in the previous study; the non-significant changes in functional capacity, and quality of life suggest that the intervention protected against the decline in functional status that is classically witnessed in the usual standard of care (63).
Knowledge gaps and future directions
It can be challenging to draw conclusions from the literature given there is a large amount of heterogeneity observed in both study populations but also the intervention protocols. The heterogeneity can be reflected by the inclusion of cancers from different anatomical locations (gastric and esophageal), but also varying clinical stages, pathologies (adenocarcinoma vs. squamous cell carcinoma), neoadjuvant treatments (NACT vs. NACRT) and surgical interventions (gastrectomy vs. esophagectomy, open vs. minimally invasive). It is important to also highlight that between each of the interventions varied significantly as per training frequency, duration, intensity, volume, and extent of qualified supervision.
The science of prehabilitation as it pertains to esophageal cancer is rapidly evolving, as such there are several components which remain unclear and warrant further exploration. Functional capacity as measured by cardiopulmonary fitness has been recognized to be an important predictive parameter of patient tolerance to NAT, but also of survival (5). To this end, a systematic review by O’Neil et al., confirmed that a low preoperative fitness was consistently associated with an increased risk of postoperative pulmonary complications (74). Exercise is recognized to be safe and feasible during NAT and provides important physiological adaptations. It improves fitness and is therefore considered an essential element of prehabilitation in esophageal cancer (65). Although the American College of Sport Medicine has published exercise guidelines for cancer patients, there is currently no consensus on the optimal exercise prescription to effectively counter the detrimental effects of neoadjuvant therapies and surgery in these patients. Other related components that warrant further investigation are different aerobic intensities (high-intensity interval training vs. moderate steady state), training location (home-based vs. supervised interventions), and IMT protocols.
Poor nutritional status and unintentional weight loss are common features of upper GI cancer, and increase patient susceptibility to postoperative morbidity and mortality (39,48). Hence, the importance of early nutritional support is universally accepted, specifically interventions recommended to ensure nutritional adequacy and oppose the depletion of physiologic reserves (39). Dietary supplements may prove to be an important utility in the nutritional management of esophageal cancer. To the same extent, supplementation with immune-modulating nutrients, protein and ergogenic aids have been reported to improve nutritional status and postoperative morbidity (19,41). The literature is however heterogeneous, and therefore there is a need for high-quality studies to identify and determine the ideal combination and dose of nutrients required to elicit a significant reduction in postoperative morbidity and mortality.
The diagnosis and management of cancer are both known to be associated with emotional distress and anxiety, which can negatively affect postoperative perception of pain and recovery. Nevertheless, very few studies have evaluated the therapeutic potential of providing these patients with psychological support prior to surgery and to what it can affect postoperative morbidity and quality of life.
Even for multimodal prehabilitation, which represents the attempt to synergize all the above components, the current level of evidence in esophageal cancer is limited, often based on retrospective study, lacking in statistical power, and focused primarily on short-term outcomes. Nevertheless, current data highlights the promising effect that prehabilitation provides for high-risk patients. Also, specifically with respect to adenocarcinoma of the esophagus, we believe there is a need for robust longitudinal studies with large sample sizes that would allow for a proper assessment of the impact it has on postoperative complications and long-term outcomes.
Conclusions
Although many clinicians agree that every effort should be made to preserve physical, nutritional, and psychological status along cancer care, data supporting the complex perioperative risk management of esophageal carcinoma resection remains insufficient.
Preventing perioperative functional decreases in cardiorespiratory reserve is of primary importance. While further studies are required to draw conclusion on surgical and long-term outcomes, mounting evidence is available on the efficacy and safety of prehabilitation on improving perioperative functional trajectories. Mirroring the philosophy of Enhanced Recovery Pathways, we suggest that prehabilitation be introduced in clinical care as a means to implement multidisciplinary and evidence-based interventions to achieve a higher standard of care for this challenging patient population.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Riccardo Rosati) for the series “Current issues on GEJ adenocarcinoma” published in Annals of Esophagus. The article has undergone external peer review.
Conflict of interest: All authors have completed the ICMJE uniform disclosure form (available at: http://dx.doi.org/10.21037/aoe-2020-15. The series “Current issues on GEJ adenocarcinoma” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 2011;29:1715-21. [Crossref] [PubMed]
- Ferri LE, Ades S, Alcindor T, et al. Perioperative docetaxel, cisplatin, and 5-fluorouracil (DCF) for locally advanced esophageal and gastric adenocarcinoma: a multicenter phase II trial. Ann Oncol 2012;23:1512-7. [Crossref] [PubMed]
- Sinclair R, Navidi M, Griffin S, et al. The impact of neoadjuvant chemotherapy on cardiopulmonary physical fitness in gastro-oesophageal adenocarcinoma. Ann R Coll Surg Engl 2016;98:396-400. [Crossref] [PubMed]
- Minnella EM, Awasthi R, Loiselle SE, et al. Effect of Exercise and Nutrition Prehabilitation on Functional Capacity in Esophagogastric Cancer Surgery: A Randomized Clinical Trial. JAMA Surg 2018;153:1081-9. [Crossref] [PubMed]
- Jack S, West MA, Raw D, et al. The effect of neoadjuvant chemotherapy on physical fitness and survival in patients undergoing oesophagogastric cancer surgery. Eur J Surg Oncol 2014;40:1313-20. [Crossref] [PubMed]
- Elliott JA, Doyle SL, Murphy CF, et al. Sarcopenia: Prevalence, and Impact on Operative and Oncologic Outcomes in the Multimodal Management of Locally Advanced Esophageal Cancer. Ann Surg 2017;266:822-30. [Crossref] [PubMed]
- Pinto E, Cavallin F, Scarpa M. Psychological support of esophageal cancer patient? J Thorac Dis 2019;11:S654-62. [Crossref] [PubMed]
- Kehlet H, Mogensen T. Hospital stay of 2 days after open sigmoidectomy with a multimodal rehabilitation programme. Br J Surg 1999;86:227-30. [Crossref] [PubMed]
- Low DE, Allum W, De Manzoni G, et al. Guidelines for Perioperative Care in Esophagectomy: Enhanced Recovery After Surgery (ERAS((R))) Society Recommendations. World J Surg 2019;43:299-330. [Crossref] [PubMed]
- Lee L, Li C, Robert N, et al. Economic impact of an enhanced recovery pathway for oesophagectomy. Br J Surg 2013;100:1326-34. [Crossref] [PubMed]
- Markar SR, Karthikesalingam A, Low DE. Enhanced recovery pathways lead to an improvement in postoperative outcomes following esophagectomy: systematic review and pooled analysis Esophagectomy enhanced recovery pathway. Dis Esophagus 2015;28:468-75. [Crossref] [PubMed]
- Chao L, Sudarshan M, Lorenzo F. Enhanced Recovery Programs for Upper Gastrointestinal Surgery: How I Do It. In: The SAGES / ERAS® Society Manual of Enhanced Recovery Programs for Gastrointestinal Surgery. 2015:313-27.
- Carli F, Zavorsky GS. Optimizing functional exercise capacity in the elderly surgical population. Curr Opin Clin Nutr Metab Care 2005;8:23-32. [Crossref] [PubMed]
- Minnella EM, Carli F. Prehabilitation and functional recovery for colorectal cancer patients. Eur J Surg Oncol 2018;44:919-26. [Crossref] [PubMed]
- O’Neill L, Guinan E, Doyle SL, et al. Rehabilitation strategies following esophageal cancer (the ReStOre trial): a feasibility study. Dis Esophagus 2017;30:1-8. [Crossref]
- Chasen MR, Bhargava R. A rehabilitation program for patients with gastroesophageal cancer—a pilot study. Support Care Cancer 2010;18:S35-40. [Crossref] [PubMed]
- Le Roy B, Selvy M, Slim K. The concept of prehabilitation: What the surgeon needs to know? J Visc Surg 2016;153:109-12. [Crossref] [PubMed]
- Jordan T, Mastnak DM, Palamar N, et al. Nutritional Therapy for Patients with Esophageal Cancer. Nutr Cancer 2018;70:23-9. [Crossref] [PubMed]
- Steenhagen E, van Vulpen JK, van Hillegersberg R, et al. Nutrition in peri-operative esophageal cancer management. Expert Rev Gastroenterol Hepatol 2017;11:663-72. [Crossref] [PubMed]
- Lagergren J, Smyth E, Cunningham D, et al. Oesophageal cancer. Lancet 2017;390:2383-96. [Crossref] [PubMed]
- Arends J, Bachmann P, Baracos V, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr 2017;36:11-48. [Crossref] [PubMed]
- Doganay E, Moorthy K. Prehabilitation for esophagectomy. J Thorac Dis 2019;11:S632-8. [Crossref] [PubMed]
- Le Roy B, Pereira B, Bouteloup C, et al. Effect of prehabilitation in gastro-oesophageal adenocarcinoma: study protocol of a multicentric, randomised, control trial—the PREHAB study. BMJ Open 2016;6:e012876. [Crossref] [PubMed]
- Mavros MN, Athanasiou S, Gkegkes ID, et al. Do Psychological Variables Affect Early Surgical Recovery? PLoS One 2011;6:e20306. [Crossref] [PubMed]
- Minnella EM, Gillis C, Edgar L, et al. Prehabilitation. In: Ljungqvist O, Francis NK, Urman RD, editors. Enhanced Recovery After Surgery - A Complete Guide to Optimizing Outcomes. Springer Nature, 2020:89-99.
- Bozzetti F. Screening the nutritional status in oncology: a preliminary report on 1,000 outpatients. Support Care Cancer 2009;17:279-84. [Crossref] [PubMed]
- Ejaz A, Spolverato G, Kim Y, et al. Impact of body mass index on perioperative outcomes and survival after resection for gastric cancer. J Surg Res 2015;195:74-82. [Crossref] [PubMed]
- Rey-Ferro M, Castano R, Orozco O, et al. Nutritional and immunologic evaluation of patients with gastric cancer before and after surgery. Nutrition 1997;13:878-81. [Crossref] [PubMed]
- Malone DL, Genuit T, Tracy JK, et al. Surgical site infections: reanalysis of risk factors. J Surg Res 2002;103:89-95. [Crossref] [PubMed]
- Ryu SW, Kim IH. Comparison of different nutritional assessments in detecting malnutrition among gastric cancer patients. World J Gastroenterol 2010;16:3310-7. [Crossref] [PubMed]
- Kondrup J, Rasmussen HH, Hamberg O, et al. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr 2003;22:321-36. [Crossref] [PubMed]
- Gabrielson DK, Scaffidi D, Leung E, et al. Use of an abridged scored Patient-Generated Subjective Global Assessment (abPG-SGA) as a nutritional screening tool for cancer patients in an outpatient setting. Nutr Cancer 2013;65:234-9. [Crossref] [PubMed]
- Anandavadivelan P, Brismar TB, Nilsson M, et al. Sarcopenic obesity: A probable risk factor for dose limiting toxicity during neo-adjuvant chemotherapy in oesophageal cancer patients. Clin Nutr 2016;35:724-30. [Crossref] [PubMed]
- Palmela C, Velho S, Agostinho L, et al. Body Composition as a Prognostic Factor of Neoadjuvant Chemotherapy Toxicity and Outcome in Patients with Locally Advanced Gastric Cancer. J Gastric Cancer 2017;17:74-87. [Crossref] [PubMed]
- Tan BH, Brammer K, Randhawa N, et al. Sarcopenia is associated with toxicity in patients undergoing neo-adjuvant chemotherapy for oesophago-gastric cancer. Eur J Surg Oncol 2015;41:333-8. [Crossref] [PubMed]
- Weimann A, Braga M, Harsanyi L, et al. ESPEN Guidelines on Enteral Nutrition: Surgery including organ transplantation. Clin Nutr 2006;25:224-44. [Crossref] [PubMed]
- McClave SA, Taylor BE, Martindale RG, et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr 2016;40:159-211. [Crossref] [PubMed]
- Licker M, Navarro R. Prehabilitation in Thoracic Surgery. Anesthesia in Thoracic Surgery. Springer, 2020:33-47.
- Anandavadivelan P, Lagergren P. Cachexia in patients with oesophageal cancer. Nat Rev Clin Oncol 2016;13:185-98. [Crossref] [PubMed]
- Bozzetti F, Arends J, Lundholm K, et al. ESPEN Guidelines on Parenteral Nutrition: non-surgical oncology. Clin Nutr 2009;28:445-54. [Crossref] [PubMed]
- Mimatsu K, Fukino N, Ogasawara Y, et al. Effects of Enteral Immunonutrition in Esophageal Cancer. Gastrointestinal tumors 2018;4:61-71. [Crossref] [PubMed]
- Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489-95. [Crossref] [PubMed]
- Campbell KL, Winters-Stone KM, Wiskemann J, et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med Sci Sports Exerc 2019;51:2375-90. [Crossref] [PubMed]
- American College of Sports M, Riebe D, Ehrman JK, et al. ACSM's guidelines for exercise testing and prescription. Tenth edition. Philadelphia: Wolters Kluwer, 2018.
- Christensen JF, Simonsen C, Hojman P. Exercise Training in Cancer Control and Treatment. Compr Physiol 2018;9:165-205. [Crossref] [PubMed]
- Fuller JT, Hartland MC, Maloney LT, et al. Therapeutic effects of aerobic and resistance exercises for cancer survivors: a systematic review of meta-analyses of clinical trials. Br J Sports Med 2018;52:1311. [Crossref] [PubMed]
- Scott JM, Zabor EC, Schwitzer E, et al. Efficacy of Exercise Therapy on Cardiorespiratory Fitness in Patients With Cancer: A Systematic Review and Meta-Analysis. J Clin Oncol 2018;36:2297-305. [Crossref] [PubMed]
- Mislang AR, Di Donato S, Hubbard J, et al. Nutritional management of older adults with gastrointestinal cancers: An International Society of Geriatric Oncology (SIOG) review paper. Journal of geriatric oncology 2018;9:382-92. [Crossref] [PubMed]
- Guinan EM, Doyle SL, Bennett AE, et al. Sarcopenia during neoadjuvant therapy for oesophageal cancer: characterising the impact on muscle strength and physical performance. Support Care Cancer 2018;26:1569-76. [PubMed]
- Simonsen C, de Heer P, Bjerre ED, et al. Sarcopenia and Postoperative Complication Risk in Gastrointestinal Surgical Oncology: A Meta-analysis. Ann Surg 2018;268:58-69. [Crossref] [PubMed]
- Scheede-Bergdahl C, Minnella EM, Carli F. Multi-modal prehabilitation: addressing the why, when, what, how, who and where next? Anaesthesia 2019;74 Suppl 1:20-6. [Crossref] [PubMed]
- Walburn J, Vedhara K, Hankins M, et al. Psychological stress and wound healing in humans: A systematic review and meta-analysis. Journal of Psychosomatic Research 2009;67:253-71. [Crossref] [PubMed]
- Tsimopoulou I, Pasquali S, Howard R, et al. Psychological Prehabilitation Before Cancer Surgery: A Systematic Review. Ann Surg Oncol 2015;22:4117-23. [Crossref] [PubMed]
- Burden S, Todd C, Hill J, et al. Pre-operative nutrition support in patients undergoing gastrointestinal surgery. Cochrane Database Syst Rev 2012;11:CD008879. [Crossref] [PubMed]
- Wong CS, Aly EH. The effects of enteral immunonutrition in upper gastrointestinal surgery: A systematic review and meta-analysis. Int J Surg 2016;29:137-50. [Crossref] [PubMed]
- Fujitani K, Tsujinaka T, Fujita J, et al. Prospective randomized trial of preoperative enteral immunonutrition followed by elective total gastrectomy for gastric cancer. Br J Surg 2012;99:621-9. [Crossref] [PubMed]
- Sultan J, Griffin SM, Di Franco F, et al. Randomized clinical trial of omega-3 fatty acid-supplemented enteral nutrition versus standard enteral nutrition in patients undergoing oesophagogastric cancer surgery. Br J Surg 2012;99:346-55. [Crossref] [PubMed]
- Song GM, Tian X, Liang H, et al. Role of Enteral Immunonutrition in Patients Undergoing Surgery for Gastric Cancer: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medicine (Baltimore) 2015;94:e1311. [Crossref] [PubMed]
- Mabvuure NT, Roman I, Khan OA. Enteral immunonutrition versus standard enteral nutrition for patients undergoing oesophagogastric resection for cancer. Int J Surg 2013;11:122-7. [Crossref] [PubMed]
- Yamana I, Takeno S, Hashimoto T, et al. Randomized Controlled Study to Evaluate the Efficacy of a Preoperative Respiratory Rehabilitation Program to Prevent Postoperative Pulmonary Complications after Esophagectomy. Dig Surg 2015;32:331-7. [Crossref] [PubMed]
- Xu YJ, Cheng JC, Lee JM, et al. A Walk-and-Eat Intervention Improves Outcomes for Patients With Esophageal Cancer Undergoing Neoadjuvant Chemoradiotherapy. Oncologist 2015;20:1216-22. [Crossref] [PubMed]
- Valkenet K, Trappenburg JC, Schippers CC, et al. Feasibility of Exercise Training in Cancer Patients Scheduled for Elective Gastrointestinal Surgery. Dig Surg 2016;33:439-47. [Crossref] [PubMed]
- Wynter-Blyth V, Halliday L, Osborn H, et al. Prehabilitation reduces the extent of functional deterioration associated with neoadjuvant chemotherapy (NAC) and surgery in patients with oesophago-gastric cancer. Clin Nutr Exp 2017;19:86.
- Valkenet K, Trappenburg JCA, Ruurda JP, et al. Multicentre randomized clinical trial of inspiratory muscle training versus usual care before surgery for oesophageal cancer. Br J Surg 2018;105:502-11. [Crossref] [PubMed]
- Christensen JF, Simonsen C, Banck-Petersen A, et al. Safety and feasibility of preoperative exercise training during neoadjuvant treatment before surgery for adenocarcinoma of the gastro-oesophageal junction. BJS Open 2018;3:74-84. [Crossref] [PubMed]
- Guinan EM, Forde C, O’Neill L, et al. Effect of preoperative inspiratory muscle training on physical functioning following esophagectomy. Dis Esophagus 2019;32:doy091. [Crossref] [PubMed]
- Halliday LJ, Doganay E, Wynter-Blyth V, et al. Adherence to Pre-operative Exercise and the Response to Prehabilitation in Oesophageal Cancer Patients. J Gastrointest Surg 2020. Epub ahead of print. [Crossref] [PubMed]
- Inoue J, Ono R, Makiura D, et al. Prevention of postoperative pulmonary complications through intensive preoperative respiratory rehabilitation in patients with esophageal cancer Prevention of pulmonary complications. Dis Esophagus 2013;26:68-74. [Crossref] [PubMed]
- Valkenet K, Trappenburg JCA, Ruurda JP, et al. Multicentre randomized clinical trial of inspiratory muscle training versus usual care before surgery for oesophageal cancer. Br J Surg 2018;105:502-11. [Crossref] [PubMed]
- Barberan-Garcia A, Ubré M, Roca J, et al. Personalised Prehabilitation in High-risk Patients Undergoing Elective Major Abdominal Surgery: A Randomized Blinded Controlled Trial. Ann Surg 2018;267:50-6. [Crossref] [PubMed]
- Zhang XD, Zhao QY, Fang Y, et al. Perioperative comprehensive supportive care interventions for Chinese patients with esophageal carcinoma: a prospective study. Asian Pac J Cancer Prev 2013;14:7359-66. [Crossref] [PubMed]
- Scarpa M, Pinto E, Saraceni E, et al. Randomized clinical trial of psychological support and sleep adjuvant measures for postoperative sleep disturbance in patients undergoing oesophagectomy. Br J Surg 2017;104:1307-14. [Crossref] [PubMed]
- Persson CR, Johansson BB, Sjoden PO, et al. A randomized study of nutritional support in patients with colorectal and gastric cancer. Nutr Cancer 2002;42:48-58. [Crossref] [PubMed]
- O’Neill L, Moran J, Guinan EM, et al. Physical decline and its implications in the management of oesophageal and gastric cancer: a systematic review. J Cancer Surviv 2018;12:601-18. [Crossref] [PubMed]
Cite this article as: Minnella EM, Drummond K, Carli F. The impact of prehabilitation on surgical outcomes. Ann Esophagus 2021;4:10.



