
食管癌切除手术后吻合口瘘的高危因素分析及手术治疗现状
背景简介
全世界范围内,食管及胃食管连接部癌是癌症相关死亡的第六位,其发生率逐渐增加[1,2]。食管切除术是治疗疾病的基石,通常会联合新辅助放化疗或围术期化疗[3,4]。最常用的手术技术是Orringer手术(经腹手术颈部吻合)、McKeown三切口手术(经胸颈部吻合)和Ivor Lewis手术(经胸胸内吻合)。所有手术都可以通过开放、微创开放联合、全微创和机器人辅助来完成。
无论手术方法如何,食管癌切除术都与发病率显著相关。从外科医生的角度来看,最可怕的并发症可能是食管胃吻合口瘘,其发生率为5%~30%[5,6]。吻合口瘘是导致发病率和死亡率升高的主要原因[6-12]。而且它与生活质量恢复和不良肿瘤预后有关[13-15]。因此,吻合口瘘的预防和最佳治疗方案的选择极其重要。
吻合口瘘的治疗方案多种多样,从非干预保守治疗使用抗生素,到有创治疗方案,如介入治疗及内镜下治疗,再到手术治疗。但是关于最佳治疗方案的决策制定缺乏良好证据支持。而最近的一项国际调研中发现不同方案间存在显著差异[16]。一项截至2017年的系统回顾性研究指出,由于样本数量少,研究间异质性、缺乏有关患者基本情况和吻合口瘘特征的相关信息,没有任何一项治疗方案有证据支持[17]。目前来说,现有的治疗吻合口瘘的方案千差万别,它们只是基于专家观点而非经过验证的治疗策略。
由于患者的临床表现差别很大,建立单一治疗方案的临床路径有些困难。比如有些患者表现为小瘘口,临床症状轻微,无明显胸内表现,而另一些则表现为吻合口大面积缺损伴暴发性脓毒症和多器官功能衰竭。因此外科医生在选择治疗方案时把个体化差异和瘘情况差异考虑在内是合理而且可以理解的。然而目前尚不清楚是哪些患者和瘘特征决定了吻合口瘘的严重程度。
这篇回顾分析有两个目的:第一,阐述影响吻合口瘘发生及其临床预后的影响因素;第二,回顾一些最新文献来了解外科手术在治疗吻合口瘘中的角色。
吻合口瘘:定义和影响因素
根据最新的食管切除并发症共识组(Esophagectomy Complications Consensus Group,ECCG)一致定义,吻合口瘘被定义为包括食管、吻合口、钉仓切割缘或管状胃在内全层胃肠道缺损,且与临床表现和识别方式无关[18]。严重吻合口瘘传统的分类方法是基于侵入性治疗的方式,由ECCG所描述,具体见表1[18]。

Full table
风险的相关因素可分为患者、肿瘤和围手术期因素(见表2)。此外,不同手术技术和干预措施被证实会影响吻合口瘘的发生(见表3)。
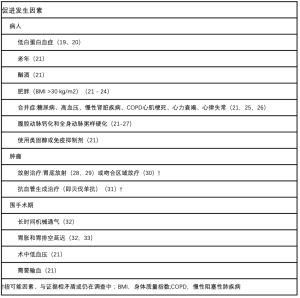
Full table

Full table
当瘘发生时,有些因素会影响预后。比如,低白蛋白血症与保守治疗失败率和恢复时间长有关系[7,39]。高龄和ASA分级也会增加吻合口瘘患者的死亡率[40]。而这些相关性可能是由于机体应激反应和分解代谢能力减退,而他们往往与吻合口瘘共存。与侵入性更强的治疗,更长住院时间、ICU时间和更长吻合口愈合时间有关的因素,包括非局限性瘘合并胸内或纵隔污染[8,39,41,42]。有一项研究报道早期瘘(<7天)和临床症状明显的瘘,与更高的死亡率和吻合口瘘发生率有关[40]。尽管大多数研究是回顾性的,在强度和样本量上存在差异使得相关性受限,因此结论是基于较差的证据等级得出的。
关于手术技术,据报道,与吻合口瘘预后有关的因素有吻合位置和大网膜加固吻合口。颈部吻合与胸内吻合相比,瘘的发生率更高。经胸McKeown手术和经腹Orringer手术均提倡颈部吻合,并接受其风险,用以作为折中方案,因为他们认为即使有瘘发生,后果也不会非常严重,在经过颈部切口引流后避免了纵隔和胸内的表现,也避免了严重脓毒症和相关死亡。尽管争议仍将持续,但一项随机对照的Meta分析显示,在忽略了吻合口方面的考虑下,McKeown 手术和Ivor Lewis手术后的总体死亡率无显著差异[13]。我们团队正在奈梅亨进行的一项研究,其目的也是对比Orringer手术与McKeown手术和Ivor Lewis手术后发生吻合口瘘的预后情况。网膜成形术作为减少吻合口瘘发生、瘘严重性、再手术风险和相关死亡的因素,虽然被部分研究[43-45]所支持,但没有得到最近一项Meta分析的证实,因此缺乏充分的证据[46]。
总之,迄今为止的证据表明患者、肿瘤和围手术期相关因素影响吻合口瘘的发生,而不同手术干预措施减少了吻合口瘘的发生。关于吻合口瘘预后差的相关因素,缺乏相关数据。深入了解这些因素对于临床医生制定有循证依据的决策来说至关重要。
吻合口瘘治疗
近几十年来吻合口瘘的干预措施稳步增多,既包括非侵入性方式也包括微侵入性治疗方式。既往有部分医生建议[47]发生瘘后立即手术,不过最近的研究表明治疗方法应该是一种渐进式方式,手术治疗应该被保留到非侵入方式和内镜与介入治疗失败之后[7,8,39,48]。下面有一些适合所有出现瘘的患者治疗的基本原则。早期发现偏离正常康复病程,并迅速诊断仍然是关键[48-50]。目前缺乏一种有循证依据诊断方法,对于在食管切除术后早期诊断瘘的患者监测其恢复的常规诊断方案尚无共识。多种临床、实验室和影像学检查可以帮助早期诊断吻合口瘘并能够早期治疗[51,52]。然而,影像学检查仅应在怀疑有吻合口瘘时进行,因为常规内镜、增强CT在没有临床怀疑的患者中并没有显示有效。一旦诊断有瘘,需要遵守的重要原则是采取充分的引流措施,并从源头控制瘘,以防止进一步扩展,控制脓毒症的进展[58]。
非侵入性治疗
保守的非侵入性治疗方法包括禁食、抗生素治疗,而放置或保留鼻胃管既可以维持引流也可以作为营养支持方式。以往的研究表明,所有患者的早期就应该使用抗生素治疗和保证持续鼻饲营养,而且最好是肠内营养[48,59]。有报道[39,41,53,60-62]指出保守治疗已经成功治愈了许多颈部及胸内吻合口瘘。Manghelli等人[39]回顾性研究了61例食管切除术后颈部或胸内瘘的患者,有46(75%)人最初接受保守治疗,仅有11人需要支架和再手术等治疗,不过这与吻合位置无关。此外,对于初始接受手术治疗和保守治疗失败后再手术治疗的两组病例,其死亡率和ICU停留时间没有显著差异。因此,尽管相关研究较少且缺乏比较研究,但当非侵入性治疗失败时,进一步采用更具侵入性的治疗方式似乎是可行和成功的。
内镜治疗
通过内镜来处理吻合口瘘的方法已有很多。内镜下在吻合口内放置引流管可以用来引流胸管或经皮引流无法引流的液体腔[61-64]。此外,对于污染局限或引流充分的患者植入完全覆盖的自膨式支架,可以防止进一步的瘘[45-47]。然而有些患者也需要再次内镜干预,比如去除支架或处理支架相关并发症。最近出现了腔内真空辅助闭合装置(E-VAC或EsoSponge),不同作者[65-67]均认为它比支架植入效果更佳,因为它既可以有效引流又有刺激吻合口愈合特点。还有部分作者[68,69]提出将E-VAC和支架结合能取得更好的结果。然而对有广泛胸内感染的患者,内镜支架植入或E-VAC治疗显得不够充分,这类病例往往需要更加充分的引流[9,70]。内镜下吻合口瘘的处理方法及其结果将在本刊的附稿中详细讨论。
手术治疗
手术治疗的目的是充分引流污染区域、手术修复吻合口或进行食管近端分流。一旦有外科干预适应症,现有证据表明及时手术很重要[71]。吻合口瘘的手术治疗包括床边胸管置入、再手术引流、再手术吻合、再手术切除吻合口并食管分流。再次手术可以通过开放(如开腹、开胸)或微创方法进行[腹腔镜、胸腔镜、电视辅助胸腔镜(VATS)入路。
手术引流
引流方式取决于污染的位置和程度,也会因吻合部位而有差别。最近的一项回顾性队列研究揭示了食管切除术后颈部吻合口瘘的预后情况[42]。有趣的是只有40%病例的瘘局限于颈部,突出表明颈部吻合后大部分吻合口可居于纵隔内。所有患者均通过手术开放颈部伤口进行引流。尽管对所有瘘至颈部的患者进行颈部伤口引流是有效的,但超过一半的患者有胸内表现且需要额外经胸引流,胸腔内污染与脓毒症的发展、ICU和住院时间延长有关。虽然有胸内表现的瘘死亡率较高,但这一发现没有统计学意义。与本研究一致,其他人也建议当瘘局限在颈部时,开放颈部伤口引流就足够了,但如果存在胸内污染,则需要进一步手术引流[8]。
针对胸内吻合口瘘,一项回顾性队列研究报告了“三管”法的结果,需放置鼻胃减压管、鼻空肠营养管和胸管[7]。它与接受手术再探查的患者相比,瘘的愈合时间较短,这种方法不但对局限于胸腔内瘘有效而对有胸膜腔严重污染并导致脓肿、纵隔感染、脓胸或脓毒症症状的非局限胸腔内瘘也是有效的。然而,在接受手术再探查的患者中,非局限性的瘘的比例较高,而对这两种治疗方法都没有明确的适应证。另外,也有人认为,当胸腔内存在积液时,可能需要放置胸管,但包裹积液通过胸管无法引流时应与胸腔镜、VATS或开胸手术的手术清创相结合[72]。综上所述,现有证据似乎支持渐进方式,即首先局部引流、内窥镜或经皮引流,随后进行手术引流和必要的探查。
手术修复和转流
渐进式的方式指手术治疗应该保留到最后,直至非手术治疗失败,对于大多数患者来说,这种方式是可行的,但一些研究建议直接选择手术治疗作为最初治疗方案。72小时内有证据的瘘,在发展成严重脓毒症之前进行缝合修复吻合口缺损是有充分理由的[71,73]。在有些病例中是需要再次吻合的,而为了确保无张力再次吻合的进行,管状胃的质量和长度非常重要。一项回顾性队列研究报道了纵隔瘘患者的预后情况,有将近40%患者再次探查作为首要治疗措施[71],他们在有脓毒症之前就快速进行了手术清创,再行吻合和食管转流手术。另一部分患者主要选择保守治疗和内镜治疗。这些患者只有在吻合口缺血、脓毒症或初始治疗失败的情况下才进行手术。研究发现,与接受保守或内窥镜治疗的患者相比,初次手术治疗的患者死亡率更高,但作者承认在这一比较中存在显著偏倚。
如果缺损不能进行一期修复,可用带蒂胸壁肌肉、大网膜、胸膜或心包脂肪进行缝合和吻合口加固[9,74]。一项研究报告了19例不同原因引起的食管瘘后进行吻合修复的结果。根据瘘位置的不同,采用膈肌、背阔肌、前锯肌或胸肌瓣进行一期或二期肌瓣修复[74]。尽管有四名患者出现呼吸功能不全,需要再次干预,术后无死亡率。除了使用自体材料修复缺损外,最近的一项研究还研究了使用牛心包补片修复吻合口瘘后的持续性颈部瘘[75]。虽然这项研究仅包括7例患者,但所有患者均痊愈,未见复发吻合口瘘的报道。虽然手术修补吻合口似乎是安全有效的,但只有一项研究比较了手术治疗的患者与保守治疗或内镜治疗患者的结果。此外,患者的病理,包括手术指征和手术操作都是不一致的,因此很难得出确切的结论。
食管切除和转流是保留用在最严重的病例中,尤其是脓毒症进展,胃组织不健康和使用不安全,部分或完全胃坏死时[39,76]。吻合口旷置和食管转流的其他适应证可能包括:大于2 cm或邻近吻合环、胸腔弥漫性污染和先前治疗失败的瘘[39,50,76,77]。一种常见的食管转流技术是施行食管末端造口术[8,76,78]。另外,双管食管胃吻合术也被提到过[47]。管胃可以在近端被切断,暂时储存在腹腔内还是被切除要取决于管胃的状况。转流手术后,恢复胃肠道连续性可能是一个主要的挑战,而管胃是首选[78]。然而,食管改道后,胃可能被切除,长度不够或不适合重建。在这些病例中,结肠间置通常优于空肠间置[76,78]。重建的时机仍有争议的,尽管有成功的早期重建的案例报道[76],但很少在6个月前进行[47,78]。作者认为只有在感染、营养和身体状况得到充分改善后才能进行重建,而非确切的某些时间[47,76,78]。
综上所述,由于纳入研究的队列小,且各研究之间存在异质性,目前的文献中没有证据支持对吻合口瘘的特殊治疗。我们建议所有患者的吻合口瘘的治疗方法包括禁食、静脉注射抗生素和充足的鼻空肠营养或空肠造口营养。此外,应充分引流,最好在颈部吻合和时开放颈部切口,也可通过介入,内镜或手术引流。只有在特殊情况下(如吻合口瘘合并管胃坏死),应再次吻合或手术切除吻合口同时行食管转流[8,42,47,48,77]。在缺乏循证依据的决策的情况下,确诊瘘时我们建议根据患者身体状况和瘘的转归情况进行个体化治疗。
正在进行的试验和实验性治疗
目前有许多关于预防瘘和改善瘘愈合的正在研究的新干预措施和试验性治疗。基于强有力的理论基础,证实了在切除前对胃缺血的预处理可以改善胃灌注。该方法包括在手术切除前至少2周分别通过腹腔镜结扎或介入动脉栓塞来处理胃左动脉[79,80]。结果因数据而异,例如,最近的一项Meta分析显示,没有证据表明吻合口瘘发生率降低,但它得出的结论是,这些干预措施是可行的,不过需要进行随机试验[36]。两项新的随机试验和一项前瞻性队列研究的结果正在等待(NCT04268654,NCT02432794,NCT03896399)[81-83]。
另外一种改善胃灌注的方法,特别是在有血管疾病的高危患者中,将管状胃动脉和邻近的胸或颈动脉之间进行血管(或微小血管)吻合。这种技术通常被称为“上段桥接”,主要用于空肠或结肠间置重建[84],但也被描述为管胃重建[37,38]。一项包括44例患者的回顾性研究表明,与常规手术相比,这种方法吻合口瘘的发生率显著降低[37]。该研究没有描述为什么选择患者进行上段桥接吻合术。尽管目前还没有定论,但增压可能在有血管疾病和常规管胃成形成后缺血风险高的患者中发挥作用。
通过术中灌注监测术中评估管胃灌注的研究越来越多[85]。最常见的评估方式是使用吲哚菁绿(ICG)进行荧光成像。最近的一项Meta分析报告称,术中ICG荧光成像导致约25%的病例(86例)的治疗改变,包括切除一部分管胃或改变吻合口位置,并且据报道使用ICG荧光成像的患者吻合口瘘概率较低。然而,与该主题的许多文献一样,报告的研究质量较差,且没有进行随机对照试验,因此大多数研究缺乏对照队列。这种方法的另一个缺陷是目前缺乏可靠的量化措施[85,86]。
干细胞治疗是治疗吻合口瘘的一种引人关注的理论方法。在中国的一项研究中[87],在动物模型中,将自体间充质干细胞混合在纤维蛋白溶液中注射到颈部吻合口瘘周围,并与单纯纤维蛋白注射进行比较,干预组创面愈合率高,创面感染率低。然而,其在人体中的安全性和有效性还有待研究。“吻合口胶”的概念并不新鲜,纤维蛋白胶(不含干细胞)以前曾被研究用于防止胃肠道吻合口瘘,特别是结肠的手术,但目前尚未被证实有效[88,89]。目前正在进行一项随机试验,旨在调查猪纤维蛋白密封剂(Bioseal)在预防McKeown食管切除术后患者吻合口瘘的有效性,结果预计在2023年取得(NCT03529266)[90]。
讨论
本综述描述了食管切除术后吻合口瘘发生和预后的相关因素和治疗方法,特别是手术治疗,以及新方法的前景。回顾现有文献,有几个因素与增加吻合口瘘的发生率有关,但与吻合口瘘的结果有关的因素所知甚少。目前,缺乏基于循证依据的治疗策略。根据现有文献,一个合理的结论是,从非侵入性治疗到更具侵入性治疗的方法提供了最好的可用方案,我们已经强调了一些需要选择立即手术作为最佳方案的例外情况。然而,研究的循证依据较弱,而且大多基于专家意见。研究应根据瘘的时间、瘘的严重程度,以及相关的脓毒性反应和器官衰竭程度建立新的策略。
这篇综述强调了现有文献的局限性。首先,大多数关于吻合口瘘治疗的研究都是回顾性的、非对比的队列研究,样本量少,随之而来会存在较大的偏倚风险。其次,研究之间存在很大的异质性,包括纳入标准和结果参数的报告,限制了研究的可比性和结论的确定性。第三,在目前的研究中,大多数患者都进行了开放食管切除术,而在目前的实践中,MIE的实施越来越多,这可能限制了这些研究结果的普及性。最后,更普遍的是,没有与临床相关同时可用于诊断的吻合口瘘严重程度的分类。尽管目前吻合口的严重程度的ECCG分类法可以用于诸多方面,同时将改进标准报告[18],但这种分类在初步诊断制定治疗方案时并不适用,因为它是根据给予的治疗对吻合口瘘进行分类的。显然,对于基于临床和瘘特征的供临床应用的瘘严重程度分类方法的需求尚未得到满足,国际网络协作有望填补这些知识的空白,并在未来提供更可靠的循证证据和策略。
在这方面,EsoBenchmark是一项由大容量的中心建立的国际倡议,以确定全微创经胸食管切除术的最佳预后[91]。esdata是由ECCG发起国际食道疾病学会(ISDE)赞助的另一项国际合作,旨在比较食道切除术后的预后[92]。最近,食管胃吻合审计组织(OGAA)发起了一项全球多中心队列研究[93]。毫无疑问,这项研究将就国际间吻合口瘘发生率的差异及吻合技术与患者预后关系的问题提供有价值的答案。然而,本研究并不能探讨与吻合口瘘预后或不同吻合口瘘治疗效果相关的因素。为了回答这两个问题,目前正在进行TENTACLE-食道研究(食管切除术后吻合口瘘的治疗)(NCT03829098)[94]。TENTACLE研究的第一个目的是调查哪些因素影响吻合口瘘的严重程度,并构建一个有循证依据的吻合口瘘严重程度评分。第二个目的是研究瘘的哪些特征与不同处理方法的成功与否相关,并根据严重程度特征对不同初始处理方法的效果进行比较。根据样本量计算,需要纳入680例患者。然而,已经有超过1 500名患者被纳入研究,结果预计在2021年初得出。
综上所述,尽管食管切除术后吻合口瘘比较常见,但目前现有文献没有提供强有力的循证依据为瘘的管理提供明确的建议,或是基于瘘的性质的脓毒症的严重程度的明确治疗方案。这提供了一个重要的研究机会,希望TENTACLE-食道和其他的研究将极大地促进知识的发展并作为吻合口瘘的治疗指南的支撑。
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Riccardo Rosati) for the series “Current issues on GEJ adenocarcinoma” published in Annals of Esophagus. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at: http://dx.doi.org/10.21037/aoe-2020-18). The series “Current issues on GEJ adenocarcinoma” was commissioned by the editorial office without any funding or sponsorship. JVR serves as an unpaid editorial board member of Annals of Esophagus from Sept 2019 to Aug 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Shapiro J, van Lanschot JJB, Hulshof MCCM, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. [Crossref] [PubMed]
- Al-Batran S-E, Homann N, Schmalenberg H, et al. Perioperative chemotherapy with docetaxel, oxaliplatin, and fluorouracil/leucovorin (FLOT) versus epirubicin, cisplatin, and fluorouracil or capecitabine (ECF/ECX) for resectable gastric or gastroesophageal junction (GEJ) adenocarcinoma (FLOT4-AIO): A multicenter, randomized phase 3 trial. J Clin Oncol 2017;35:4004. [Crossref]
- Dutch Upper GI Cancer Audit (DUCA): Analysis of DUCA registration database. 2012.
- Biere SS, Maas KW, Cuesta MA, et al. Cervical or thoracic anastomosis after esophagectomy for cancer: a systematic review and meta-analysis. Dig Surg 2011;28:29-35. [Crossref] [PubMed]
- Guo J, Chu X, Liu Y, et al. Choice of therapeutic strategies in intrathoracic anastomotic leak following esophagectomy. World J Surg Oncol 2014;12:402. [Crossref] [PubMed]
- Turkyilmaz A, Eroglu A, Aydin Y, et al. The management of esophagogastric anastomotic leak after esophagectomy for esophageal carcinoma. Dis Esophagus 2009;22:119-26. [Crossref] [PubMed]
- Schaheen L, Blackmon SH, Nason KS. Optimal approach to the management of intrathoracic esophageal leak following esophagectomy: a systematic review. Am J Surg 2014;208:536-43. [Crossref] [PubMed]
- Goense L, van Dijk WA, Govaert JA, et al. Hospital costs of complications after esophagectomy for cancer. Eur J Surg Oncol 2017;43:696-702. [Crossref] [PubMed]
- Lubbers M, van Workum F, Berkelmans G. Treatment of anastomotic leakage after minimally invasive Ivor Lewis esophagectomy. Submitted 2019.
- Saluja SS, Ray S, Pal S, et al. Randomized trial comparing side-to-side stapled and hand-sewn esophagogastric anastomosis in neck. J Gastrointest Surg 2012;16:1287-95. [Crossref] [PubMed]
- Derogar M, Orsini N, Sadr-Azodi O, et al. Influence of major postoperative complications on health-related quality of life among long-term survivors of esophageal cancer surgery. J Clin Oncol 2012;30:1615-9. [Crossref] [PubMed]
- Scarpa M, Saadeh LM, Fasolo A, et al. Health-related quality of life in patients with oesophageal cancer: analysis at different steps of the treatment pathway. J Gastrointest Surg 2013;17:421-33. [Crossref] [PubMed]
- Fransen LFC, Berkelmans GHK, Asti E, et al. The Effect of Postoperative Complications After Minimally Invasive Esophagectomy on Long-term Survival: An International Multicenter Cohort Study. Ann Surg 2020. Epub ahead of print. [Crossref] [PubMed]
- Hagens ERC, Anderegg MCJ, van Berge Henegouwen MI, et al. International Survey on the Management of Anastomotic Leakage After Esophageal Resection. Ann Thorac Surg 2018;106:1702-8. [Crossref] [PubMed]
- Verstegen MHP, Bouwense SAW, van Workum F, et al. Management of intrathoracic and cervical anastomotic leakage after esophagectomy for esophageal cancer: a systematic review. World J Emerg Surg 2019;14:17. [Crossref] [PubMed]
- Low DE, Alderson D, Cecconello I, et al. International Consensus on Standardization of Data Collection for Complications Associated With Esophagectomy: Esophagectomy Complications Consensus Group (ECCG). Ann Surg 2015;262:286-94. [Crossref] [PubMed]
- Tabatabai A, Hashemi M, Mohajeri G, et al. Incidence and risk factors predisposing anastomotic leak after transhiatal esophagectomy. Ann Thorac Med 2009;4:197-200. [Crossref] [PubMed]
- Ryan AM, Hearty A, Prichard RS, et al. Association of hypoalbuminemia on the first postoperative day and complications following esophagectomy. J Gastrointest Surg 2007;11:1355-60. [Crossref] [PubMed]
- Kamarajah SK, Lin A, Tharmaraja T, et al. Risk factors and outcomes associated with anastomotic leaks following esophagectomy: a systematic review and meta-analysis. Dis Esophagus 2020;33:doz089. [Crossref] [PubMed]
- Hasegawa T, Kubo N, Ohira M, et al. Impact of body mass index on surgical outcomes after esophagectomy for patients with esophageal squamous cell carcinoma. J Gastrointest Surg 2015;19:226-33. [Crossref] [PubMed]
- Blom RL, Lagarde SM, Klinkenbijl JH, et al. A high body mass index in esophageal cancer patients does not influence postoperative outcome or long-term survival. Ann Surg Oncol 2012;19:766-71. [Crossref] [PubMed]
- Mengardo V, Pucetti F, Mc Cormack O, et al. The impact of obesity on esophagectomy: a meta-analysis. Dis Esophagus 2018.31. [Crossref] [PubMed]
- Kassis ES, Kosinski AS, Ross P Jr, et al. Predictors of anastomotic leak after esophagectomy: an analysis of the society of thoracic surgeons general thoracic database. Ann Thorac Surg 2013;96:1919-26. [Crossref] [PubMed]
- Li SJ, Wang ZQ, Li YJ, et al. Diabetes mellitus and risk of anastomotic leakage after esophagectomy: a systematic review and meta-analysis. Dis Esophagus 2017;30:1-12. [Crossref] [PubMed]
- Goense L, van Rossum PSN, Weijs TJ, et al. Aortic Calcification Increases the Risk of Anastomotic Leakage After Ivor-Lewis Esophagectomy. Ann Thorac Surg 2016;102:247-52. [Crossref] [PubMed]
- Goense L, van Rossum PSN, Ruurda JP, et al. Radiation to the Gastric Fundus Increases the Risk of Anastomotic Leakage After Esophagectomy. Ann Thorac Surg 2016;102:1798-804. [Crossref] [PubMed]
- Vande Walle C, Ceelen WP, Boterberg T, et al. Anastomotic complications after Ivor Lewis esophagectomy in patients treated with neoadjuvant chemoradiation are related to radiation dose to the gastric fundus. Int J Radiat Oncol Biol Phys 2012;82:e513-9. [Crossref] [PubMed]
- Juloori A, Tucker SL, Komaki R, et al. Influence of preoperative radiation field on postoperative leak rates in esophageal cancer patients after trimodality therapy. J Thorac Oncol 2014;9:534-40. [Crossref] [PubMed]
- Cunningham D, Stenning SP, Smyth EC, et al. Peri-operative chemotherapy with or without bevacizumab in operable oesophagogastric adenocarcinoma (UK Medical Research Council ST03): primary analysis results of a multicentre, open-label, randomised phase 2–3 trial. Lancet Oncol 2017;18:357-70. [Crossref] [PubMed]
- Cassivi SD. Leaks, strictures, and necrosis: a review of anastomotic complications following esophagectomy. Semin Thorac Cardiovasc Surg 2004;16:124-32. [Crossref] [PubMed]
- Dewar L, Gelfand G, Finley RJ, et al. Factors affecting cervical anastomotic leak and stricture formation following esophagogastrectomy and gastric tube interposition. Am J Surg 1992;163:484-9. [Crossref] [PubMed]
- Deng XF, Liu QX, Zhou D, et al. Hand-sewn vs linearly stapled esophagogastric anastomosis for esophageal cancer: a meta-analysis. World J Gastroenterol 2015;21:4757-64. [Crossref] [PubMed]
- Honda M, Kuriyama A, Noma H, et al. Hand-sewn versus mechanical esophagogastric anastomosis after esophagectomy: a systematic review and meta-analysis. Ann Surg 2013;257:238-48. [Crossref] [PubMed]
- Heger P, Blank S, Diener MK, et al. Gastric Preconditioning in Advance of Esophageal Resection-Systematic Review and Meta-Analysis. J Gastrointest Surg 2017;21:1523-32. [Crossref] [PubMed]
- Yoshimi F, Asato Y, Ikeda S, et al. Using the supercharge technique to additionally revascularize the gastric tube after a subtotal esophagectomy for esophageal cancer. Am J Surg 2006;191:284-7. [Crossref] [PubMed]
- Murakami M, Sugiyama A, Ikegami T, et al. Additional microvascular anastomosis in reconstruction after total esophagectomy for cervical esophageal carcinoma. Am J Surg 1999;178:263-6. [Crossref] [PubMed]
- Manghelli JL, Ceppa DP, Greenberg JW, et al. Management of anastomotic leaks following esophagectomy: when to intervene? J Thorac Dis 2019;11:131-7. [Crossref] [PubMed]
- Alanezi K, Urschel JD. Mortality secondary to esophageal anastomotic leak. Ann Thorac Cardiovasc Surg 2004;10:71-5. [PubMed]
- Korst RJ, Port JL, Lee PC, et al. Intrathoracic manifestations of cervical anastomotic leaks after transthoracic esophagectomy for carcinoma. Ann Thorac Surg 2005;80:1185-90. [Crossref] [PubMed]
- van Rossum PSN, Haverkamp L, Carvello M, et al. Management and outcome of cervical versus intrathoracic manifestation of cervical anastomotic leakage after transthoracic esophagectomy for cancer. Dis Esophagus 2017;30:1-8. [PubMed]
- Sepesi B, Swisher SG, Walsh GL, et al. Omental reinforcement of the thoracic esophagogastric anastomosis: an analysis of leak and reintervention rates in patients undergoing planned and salvage esophagectomy. J Thorac Cardiovasc Surg 2012;144:1146-50. [Crossref] [PubMed]
- Zhou D, Liu QX, Deng XF, et al. Anastomotic reinforcement with omentoplasty reduces anastomotic leakage for minimally invasive esophagectomy with cervical anastomosis. Cancer Manag Res 2018;10:257-63. [Crossref] [PubMed]
- Zheng QF, Wang JJ, Ying MG, et al. Omentoplasty in preventing anastomotic leakage of oesophagogastrostomy following radical oesophagectomy with three-field lymphadenectomy. Eur J Cardiothorac Surg 2013;43:274-8. [Crossref] [PubMed]
- Yuan Y, Zeng X, Hu Y, et al. Omentoplasty for esophagogastrostomy after esophagectomy. Cochrane Database Syst Rev 2012;11:CD008446. [PubMed]
- Page RD, Shackcloth MJ, Russell GN, et al. Surgical treatment of anastomotic leaks after oesophagectomy. Eur J Cardiothorac Surg 2005;27:337-43. [Crossref] [PubMed]
- Dent B, Griffin SM, Jones R, et al. Management and outcomes of anastomotic leaks after oesophagectomy. Br J Surg 2016;103:1033-8. [Crossref] [PubMed]
- Moon SW, Kim JJ, Cho DG, et al. Early detection of complications: anastomotic leakage. J Thorac Dis 2019;11:S805-11. [Crossref] [PubMed]
- Mardin WA, Palmes D, Bruewer M. Current concepts in the management of leakages after esophagectomy. Thoracic Cancer 2012;3:117-24. [Crossref] [PubMed]
- Palmes D, Bruwer M, Bader FG, et al. Diagnostic evaluation, surgical technique, and perioperative management after esophagectomy: consensus statement of the German Advanced Surgical Treatment Study Group. Langenbecks Arch Surg 2011;396:857-66. [Crossref] [PubMed]
- de Mooij CM, Maassen van den Brink M, Merry A, et al. Systematic Review of the Role of Biomarkers in Predicting Anastomotic Leakage Following Gastroesophageal Cancer Surgery. J Clin Med 2019.8. [Crossref] [PubMed]
- Griffin SM, Lamb PJ, Dresner SM, et al. Diagnosis and management of a mediastinal leak following radical oesophagectomy. Br J Surg 2001;88:1346-51. [Crossref] [PubMed]
- Nederlof N, de Jonge J, de Vringer T, et al. Does Routine Endoscopy or Contrast Swallow Study After Esophagectomy and Gastric Tube Reconstruction Change Patient Management? J Gastrointest Surg 2017;21:251-8. [Crossref] [PubMed]
- Doerfer J, Meyer T, Klein P, et al. The importance of radiological controls of anastomoses after upper gastrointestinal tract surgery - a retrospective cohort study. Patient Saf Surg 2010;4:17. [Crossref] [PubMed]
- Hogan BA, Winter D, Broe D, et al. Prospective trial comparing contrast swallow, computed tomography and endoscopy to identify anastomotic leak following oesophagogastric surgery. Surg Endosc 2008;22:767-71. [Crossref] [PubMed]
- Jones CM, Clarke B, Heah R, et al. Should routine assessment of anastomotic integrity be undertaken using radiological contrast swallow after oesophagectomy with intra-thoracic anastomosis? Best evidence topic (BET). Int J Surg 2015;20:158-62. [Crossref] [PubMed]
- Tang H, Xue L, Hong J, et al. A method for early diagnosis and treatment of intrathoracic esophageal anastomotic leakage: prophylactic placement of a drainage tube adjacent to the anastomosis. J Gastrointest Surg 2012;16:722-7. [Crossref] [PubMed]
- Elsayed H, Shaker H, Whittle I, et al. The impact of systemic fungal infection in patients with perforated oesophagus. Ann R Coll Surg Engl 2012;94:579-84. [Crossref] [PubMed]
- Qin J, Li Y, Zhang R, et al. Treatment of esophagogastric anastomotic leak with perianastomotic drain. J Thorac Oncol 2010;5:251-3. [Crossref] [PubMed]
- Hu Z, Yin R, Fan X, et al. Treatment of intrathoracic anastomotic leak by nose fistula tube drainage after esophagectomy for cancer. Dis Esophagus 2011;24:100-7. [Crossref] [PubMed]
- Jiang F, Yu MF, Ren BH, et al. Nasogastric placement of sump tube through the leak for the treatment of esophagogastric anastomotic leak after esophagectomy for esophageal carcinoma. J Surg Res 2011;171:448-51. [Crossref] [PubMed]
- Shuto K, Kono T, Akutsu Y, et al. Naso-esophageal extraluminal drainage for postoperative anastomotic leak after thoracic esophagectomy for patients with esophageal cancer. Dis Esophagus 2017;30:1-9. [PubMed]
- Yin G, Xu Q, Chen S, et al. Fluoroscopically guided three-tube insertion for the treatment of postoperative gastroesophageal anastomotic leakage. Korean J Radiol 2012;13:182-8. [Crossref] [PubMed]
- Bludau M, Holscher AH, Herbold T, et al. Management of upper intestinal leaks using an endoscopic vacuum-assisted closure system (E-VAC). Surg Endosc 2014;28:896-901. [Crossref] [PubMed]
- Rausa E, Asti E, Aiolfi A, et al. Comparison of endoscopic vacuum therapy versus endoscopic stenting for esophageal leaks: systematic review and meta-analysis. Dis Esophagus 2018.31. [Crossref] [PubMed]
- Brangewitz M, Voigtlander T, Helfritz FA, et al. Endoscopic closure of esophageal intrathoracic leaks: stent versus endoscopic vacuum-assisted closure, a retrospective analysis. Endoscopy 2013;45:433-8. [Crossref] [PubMed]
- Chon SH, Bartella I, Burger M, et al. VACStent: a new option for endoscopic vacuum therapy in patients with esophageal anastomotic leaks after upper gastrointestinal surgery. Endoscopy 2020;52:E166-7. [Crossref] [PubMed]
- Valli PV, Mertens JC, Kroger A, et al. Stent-over-sponge (SOS): a novel technique complementing endosponge therapy for foregut leaks and perforations. Endoscopy 2018;50:148-53. [Crossref] [PubMed]
- Kauer WKH, Stein HJ, Dittler H-J, et al. Stent implantation as a treatment option in patients with thoracic anastomotic leaks after esophagectomy. Surg Endosc 2008;22:50-3. [Crossref] [PubMed]
- Fumagalli U, Baiocchi GL, Celotti A, et al. Incidence and treatment of mediastinal leakage after esophagectomy: Insights from the multicenter study on mediastinal leaks. World J Gastroenterol 2019;25:356-66. [Crossref] [PubMed]
- Ding N, Mao Y, He J, et al. Experiences in the management of anastomotic leakages and analysis of the factors affecting leakage healing in patients with esophagogastric junction cancer. J Thorac Dis 2017;9:386-91. [Crossref] [PubMed]
- Messager M, Warlaumont M, Renaud F, et al. Recent improvements in the management of esophageal anastomotic leak after surgery for cancer. Eur J Surg Oncol 2017;43:258-69. [Crossref] [PubMed]
- Kotzampassakis N, Christodoulou M, Krueger T, et al. Esophageal leaks repaired by a muscle onlay approach in the presence of mediastinal sepsis. Ann Thorac Surg 2009;88:966-72. [Crossref] [PubMed]
- Hua X, Qian R, Shi K, et al. Effectiveness and safety of bovine pericardium patch repair for cervical anastomotic leakage after oesophagectomy for cancer. J Thorac Dis 2019;11:3808-13. [Crossref] [PubMed]
- Wang H, Zhang Y, Zhang Y, et al. Practice of cervical end-esophageal exteriorization in patients with severe intrathoracic anastomotic leakage after esophagectomy. J Int Med Res 2018;46:5090-8. [Crossref] [PubMed]
- Martin LW, Swisher SG, Hofstetter W, et al. Intrathoracic leaks following esophagectomy are no longer associated with increased mortality. Ann Surg 2005;242:392-9. [PubMed]
- Barkley C, Orringer MB, Iannettoni MD, et al. Challenges in reversing esophageal discontinuity operations. Ann Thorac Surg 2003;76:989-94; discussion 995. [Crossref] [PubMed]
- Yetasook AK, Leung D, Howington JA, et al. Laparoscopic ischemic conditioning of the stomach prior to esophagectomy. Dis Esophagus 2013;26:479-86. [Crossref] [PubMed]
- Diana M, Hübner M, Vuilleumier H, et al. Redistribution of Gastric Blood Flow by Embolization of Gastric Arteries Before Esophagectomy. Ann Thorac Surg 2011;91:1546-51. [Crossref] [PubMed]
- Trial on Delay Phenomenon Utility in Preventing Anastomotic Leakage After an Esophagectomy (APIL_2013) (NCT02432794). 2015. Available online: https://clinicaltrials.gov/ct2/show/NCT02432794
- Laparoscopic Ischemic Conditioning Prior to Esophagectomy (ISCON) (NCT03896399). 2019. Available online: https://clinicaltrials.gov/ct2/show/NCT03896399
- Ischemic Conditioning of the Gastric Conduit in Esophageal Cancer (NCT04268654). 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT04268654
- Sekido M, Yamamoto Y, Minakawa H, et al. Use of the “supercharge” technique in esophageal and pharyngeal reconstruction to augment microvascular blood flow. Surgery 2003;134:420-4. [Crossref] [PubMed]
- Jansen SM, de Bruin DM, van Berge Henegouwen MI, et al. Optical techniques for perfusion monitoring of the gastric tube after esophagectomy: a review of technologies and thresholds. Dis Esophagus 2018.31. [Crossref] [PubMed]
- Slooter MD, Eshuis WJ, Cuesta MA, et al. Fluorescent imaging using indocyanine green during esophagectomy to prevent surgical morbidity: a systematic review and meta-analysis. J Thorac Dis 2019;11:S755-65. [Crossref] [PubMed]
- Xue X, Yan Y, Ma Y, et al. Stem-Cell Therapy for Esophageal Anastomotic Leakage by Autografting Stromal Cells in Fibrin Scaffold. Stem Cells Transl Med 2019;8:548-56. [Crossref] [PubMed]
- Huh JW, Kim HR, Kim YJ. Anastomotic leakage after laparoscopic resection of rectal cancer: the impact of fibrin glue. Am J Surg 2010;199:435-41. [Crossref] [PubMed]
- Nordentoft T, Pommergaard HC, Rosenberg J, et al. Fibrin Glue Does Not Improve Healing of Gastrointestinal Anastomoses: A Systematic Review. Eur Surg Res 2015;54:1-13. [Crossref] [PubMed]
- Study of Porcine Fibrin Sealant in Preventing Cervical Anastomotic Leakage for Esophageal or Junctional Carcinoma (PLACE020) (NCT03529266). 2019. Available online: https://clinicaltrials.gov/ct2/show/NCT03529266
- Schmidt HM, Gisbertz SS, Moons J, et al. Defining Benchmarks for Transthoracic Esophagectomy: A Multicenter Analysis of Total Minimally Invasive Esophagectomy in Low Risk Patients. Ann Surg 2017;266:814-21. [Crossref] [PubMed]
- Low DE, Kuppusamy MK, Alderson D, et al. Benchmarking Complications Associated with Esophagectomy. Ann Surg 2019;269:291-8. [Crossref] [PubMed]
- Evans RPT, Singh P, Nepogodiev D, et al. Study protocol for a multicenter prospective cohort study on esophagogastric anastomoses and anastomotic leak (the Oesophago-Gastric Anastomosis Audit/OGAA). Dis Esophagus 2020.33. [PubMed]
- TreatmENT of AnastomotiC Leakage After Esophagectomy (TENTACLE study) (NCT03829098). 2019. Available online: https://clinicaltrials.gov/ct2/show/NCT03829098

龙涛
江苏大学硕士研究生,镇江市第一人民医院心胸外科住院医师。长期从事心胸外科疾病诊治。临床技能熟练,且参加了多项食管和肺相关国家自然科学基金、江苏省科学自然基金等多项科技计划。近年来完成多篇中英文专业论文。共同第一作者发表SCI论文一篇。(更新时间:2021/8/5)

田东
四川大学华西医院胸外科,副主任医师,硕士研究生导师。日本东京大学外科学博士(肺外科);四川大学外科学博士(食管外科);美国UTMC访问学者;加拿大多伦多总医院肺移植Training Fellow;日本京都大学Research Fellow。国际肺癌研究协会会员(IASLC),国际食管疾病学会和欧洲食管疾病学会会员(ISDE + ESDE),亚洲胸心血管外科协会会员(ASCVTS),四川省胸心血管外科青年委员,Journal of Thoracic Disease杂志,《中华肿瘤杂志》及《临床与病理杂志》编委,多本SCI收录杂志同行评审专家;美国胸外科协会(AATS)Graham Training Fellowship获得者,国家留学基金委高水平大学公派博士研究生奖学金获得者,中日笹川医学奖学金获得者,中华医学会胸心血管外科全国优秀论文二等奖获得者。(更新时间2022/7/18)
(本译文仅供学术交流,实际内容请以英文原文为准。)
Cite this article as: Ubels S, Verstegen MHP, Rosman C, Reynolds JV. Anastomotic leakage after esophagectomy for esophageal cancer: risk factors and operative treatment. Ann Esophagus 2021;4:8.


