
Optimization of detection of residual disease after neoadjuvant therapy in patients with esophageal cancer
Introduction
Standard treatment for locally advanced esophageal cancer comprises neoadjuvant therapy followed by esophagectomy (1-3). Neoadjuvant chemoradiotherapy (nCRT) according to the CROSS regimen has improved 5-year overall survival as well as the radical resection rate (3,4). Further analysis showed a pathologically complete response (pCR) in 23% of adenocarcinoma patients and 49% of squamous cell carcinoma patients treated with nCRT (3). Patients with a clinically complete response (cCR: no evidence of residual disease with diagnostics) after nCRT could possibly benefit from an active surveillance strategy: surgery is offered only to patients with evidence of residual cancer. A subgroup of patients might thus avoid the risks of postoperative mortality, morbidity, and decreased quality of life that are associated with surgery (5). The possible benefit of active surveillance strategies will become apparent from two randomized controlled SANO and ESOSTRATE trials (6,7).
In view of active surveillance, accurate clinical response evaluations (CREs) are important to timely detect residual tumor after nCRT. The composition of diagnostic modalities in CREs has been investigated previously in the Dutch diagnostic preSANO trial (8). The investigated set of diagnostic modalities has a 90% sensitivity for detection of substantial residual tumor (>10% residual viable tumor) and consists of bite-on-bite biopsies, endoscopic ultrasound with fine-needle aspiration (EUS-FNA) of suspected lymph nodes and 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT). In Asia, a similar diagnostic trial, the preSINO trial, is currently performed to investigate the composition of diagnostics in CREs for esophageal squamous cell carcinoma patients (9).
Detection of any residual locoregional tumor (≥1% residual viable tumor) with this set of modalities is less accurate, with a sensitivity of 77% and a specificity of 72%, and could be further optimized (8). Ideally, patients with any residual locoregional tumor without distant metastases are referred to immediate surgery when having a good performance status. This requires sensitive locoregional tumor detection and detection of distant metastases before surgery. At the same time, the rate of false-positive CREs should be minimized to avoid patients from undergoing unnecessary surgery. Another important aim is to minimize the burden of CREs as much as possible. High accuracy for tumor detection should be reached with a minimum number of (minimally-invasive) diagnostics, perhaps complemented with new diagnostic tests, for instance with biomarkers derived from imaging, blood samples or breath analysis.
The aim of this narrative literature review is to address the current state of diagnostic modalities used in CREs and to describe future perspectives to optimize tumor detection after nCRT for locally advanced esophageal cancer.
Endoscopic assessment
Current endoscopic procedures
Regular endoscopy with bite-on-bite biopsies and EUS-FNA of suspected lymph nodes are important modalities of current CREs, since these procedures allow for (cyto)histologic assessment of locoregional residual tumor. The highest chance of detecting residual tumor at the primary tumor site is when the mucosa and submucosa are adequately sampled. In one study, residual tumor in the resection specimen after CROSS has been reported to be located at least partially in the mucosa or submucosa in 89% of patients (10).
Detection of residual tumor has improved with application of bite-on-bite biopsies as compared to regular biopsies (8). Bite-on-bite biopsies consist of two subsequent biopsies at the same location, to get a deeper sample with the second biopsy. The hypothesis is that by taking bite-on-bite biopsies one is able to sample the submucosa. In the preSANO trial, 84 patients underwent regular biopsies and 123 patients underwent bite-on-bite biopsies. Bite-on-bite biopsies were taken at four different locations within the initial tumor site and at any other suspected site in the esophagus. In 26 patients with residual TRG3–4 disease who underwent regular biopsies, 8 of 26 patients were missed (31% false-negatives). In 41 patients with residual TRG3–4 disease who underwent bite-on-bite biopsies, 7 patients were missed (17% false-negatives). Sensitivity further improved when bite-on-bite biopsies were combined with EUS-FNA of suspected lymph nodes (4 of 41 were missed; 10% false-negatives).
Location of missed residual tumor after nCRT
Nonetheless, the rate of false-negative biopsies should ideally be further reduced, which depends on the location of missed residual tumor during CREs. This has been investigated for esophageal squamous cell carcinoma patients in one study (11). Some 41 patients were included who obtained a cCR after nCRT based on endoscopic and radiologic assessment, but who had ≥1% residual tumor at the primary tumor site in the resection specimen. Of them, 28 patients (68%) had involvement of the mucosa and 9 patients (22%) had residual tumor underneath a cancer-free mucosa. Four patients (10%) had residual tumor underneath a non-tumorous mucosa and submucosa.
Findings were comparable to results of a side-study of the preSANO trial, that predominantly included adenocarcinoma patients (van der Wilk B, 2020, personal communication). The location of missed residual tumor after nCRT was examined in 27 resection specimens, in which residual tumor had not been detected with regular biopsies with EUS-FNA or bite-on-bite biopsies with EUS-FNA. Residual disease in the resection specimen was present in at least the mucosa in 18 of 27 patients (67%). Some 8 of 27 (30%) patients had residual cancer in the submucosa under a normal mucosa. Residual disease underneath the mucosa and submucosa was found in only 1 patient (4%).
Furthermore, this study showed that the site where a biopsy is taken is difficult to determine. Histopathologic examination showed that the mucosa could be defined in 72% of the biopsy specimen collected during CREs in patients with undetected residual tumor. Specific submucosal structures could be defined in only 6%. In the remaining 21% the origin of tissue was uncertain. Moreover, examination of non-irradiated parts of the esophagus in three resection specimens showed that specific submucosal structures are present in only 1–2% of the entire submucosal area (van der Wilk B, 2020, personal communication).
The observations in these studies suggest that larger mucosal areas, within the site of the initial tumor, should be sufficiently sampled (i.e., with a minimum of four bite-on-bite biopsies according to the preSANO trial) in combination with deeper biopsies to overcome sampling errors. Evaluation of the exact biopsy depth cannot rely solely on recognition of (sub)mucosal structures after nCRT.
Wide-area transepithelial sampling with computer-assisted three-dimensional analysis (WATS-3D)
A new technique to improve sampling of large areas of the esophagus could be WATS-3D (CDx Diagnostics, Suffern, NY, USA) (12). WATS-3D obtains cytohistological and histological tissue samples from the esophagus with a brush. The obtained tissue is suitable for 3D visualization and automated detection of dysplasia by image processing using artificial intelligence (13). The brush allows for sampling of larger areas compared to regular biopsies and reaches up to the lamina propria (12).
WATS has been demonstrated to be safe in a setting of screening or surveillance for Barrett esophagus or dysplasia (12). However, it has not been tested in esophageal cancer patients after radiotherapy, nor has been compared with regular biopsies in relation to residual tumor in resection specimens. Whether WATS is of potential use in patients with esophageal cancer needs to be determined.
Other techniques that ensure deeper sampling, at least of the submucosa, remain undetermined for esophageal cancer. A fine-needle-biopsy (FNB) of the esophagus might be proposed for this purpose in future studies (14).
EUS and EUS-guided FNA
Several studies have reported that the EUS-based measurements of maximum tumor thickness and maximum tumor area are useful to predict residual tumor after nCRT (15-19). One prospective multicenter study investigated both EUS-based measurements to detect residual tumor at 12 weeks after nCRT (19). Residual tumor thickness, with a cut-off value of 4.5 mm, and residual tumor area, with a cut-off value of 0.92 cm2, were able to detect TRG3–4 tumor with a sensitivity of almost 90%. However, identification of TRG1 was less accurate, as reflected by specificities of 52% and 40% for residual tumor thickness and area respectively. Nonetheless, these EUS-based measurements might improve selection of patients for active surveillance, if further explored in combination with other diagnostic modalities.
The number of detected positive lymph nodes during CREs could be improved. Standard criteria for EUS to define suspicious lymph nodes seem inaccurate after nCRT, as was shown in a post-hoc analysis of the preSANO trial (20). In this study, about half of true-positive lymph nodes (as confirmed in the resection specimen) did not meet all official EUS criteria to be considered suspicious lymph nodes (i.e., round, hypoechogenic, and >5 mm). Despite the broadened criteria for suspicious lymph nodes used in this study, the sensitivity of EUS alone at 12 weeks after nCRT was 50% with a specificity of 78%. Moreover, of 19 patients who underwent EUS-guided FNA, FNA results were inconclusive in 8 patients. To improve accuracy of EUS-FNA, the authors of the study suggested to sample all visible lymph nodes, regardless of criteria for suspiciousness. Besides, specific attention should be paid to lymph node stations below the diaphragm, where malignant lymph nodes were most often present in this series mainly consisting of patients with an adenocarcinoma. In addition, inconclusive FNA results might be prevented by performing repeated needle-passes per lymph node or by using cytology with rapid on-site examination.
Elastography maps the stiffness of tissue in color maps on endosonographic images. This technique portrays rigid areas—such as malignant tissue—with a different color than areas of intermediate tissue elasticity or soft tissues (21). Several studies have successfully applied pre-treatment EUS elastography to detect cyto- or histopathologically confirmed positive lymph nodes (22-24). However, EUS elastography has not yet been investigated in patients after nCRT, when the elasticity of lymph nodes might have altered as a result of nCRT. The ability to discriminate for example fibrotic from malignant lymph nodes with EUS elastographic evaluation after nCRT is therefore unknown. Furthermore, contrast-enhanced harmonic EUS (CEH-EUS) might complement standard EUS. Qualitative CEH-EUS characterizes tissue according to the level of contrast enhancement (e.g., non-enhanced or hyperenhanced), which has been described valuable for diagnosing pancreatic lesions (25). At present, no studies have evaluated CEH-EUS for initial esophageal cancer staging or response assessment. The diagnostic value of CEH-EUS in esophageal cancer thus remains uncertain.
Radiologic and nuclear imaging techniques
18F-FDG PET/CT
18F-FDG PET/CT has been extensively studied for response assessment during and after nCRT in esophageal cancer, without satisfactory results for the detection of residual tumor at the primary tumor site. A meta-analysis reported a pooled sensitivity of 74% [95% confidence interval (CI): 0.68–0.79] and pooled specificity of 52% (95% CI: 0.44–0.60) for detecting ypT0/ypT0N0 with qualitative 18F-FDG PET (26). The pooled sensitivity was consistent with results of in-depth analyses of the preSANO trial, in which qualitative 18F-FDG PET/CT analysis at 12 weeks after nCRT had a sensitivity of 80% for ypT0 (27). However, the specificity was low due to 63% false-positives, probably because of persisting post-radiation esophagitis at 12 weeks after nCRT.
The current role of 18F-FDG PET/CT during CREs is mainly to guide EUS-FNA by identification of 18F-FDG positive lymph nodes and to detect intercurrent hematogenous metastases. Serial 18F-FDG PET/CT during active surveillance seems promising for detection of tumor recurrence at the primary tumor site (Valkema M, 2020, personal communication). A retrospective analysis was performed on a cohort of patients with cCR after nCRT who declined standard surgery and underwent active surveillance with serial 18F-FDG PET/CT scans. In patients without biopsy-proven tumor recurrence during active surveillance, 18F-FDG uptake at the primary tumor site continued to decrease beyond 12 weeks after nCRT, which was indicative of recovery from post-radiation esophagitis. By contrast, patients who developed biopsy-proven recurrence beyond 12 weeks after nCRT had an increasing local 18F-FDG uptake. These findings indicate that 18F-FDG PET/CT might be valuable to monitor local tumor response during active surveillance, but this needs to be confirmed in a prospective setting.
Magnetic resonance imaging (MRI)
MRI offers high soft tissue resolution and is able to depict esophageal layers and tumor invasion depth (28,29). Tissue on MRI is characterized with T1 and T2-weighted images and with additional MRI techniques such as diffusion-weighted MRI (DWI or DW-MRI) and dynamic contrast-enhanced MRI (DCE-MRI). DWI provides functional information about the movement of water molecules in tissue, based on the cell density and the integrity of cell membranes. DWI is quantified using the apparent diffusion coefficient (ADC). ADC values are low in malignant tissue, reflecting high cellular density resulting in diffusion restriction of water molecules, whereas the ADC values are typically high in non-cancerous tissue, where cellular density is lower (30,31). Besides DWI, DCE-MRI can be performed after administration of intravenous contrast. DCE-MRI may reflect altered tissue vascularity in malignant tissue as opposed to healthy tissue (32). The combination of DWI and DCE-MRI might be of value in esophageal cancer, since quantitative DWI and DCE-MRI parameters have shown complementary value in prediction of pCR after nCRT (33).
ADC values tend to be increased in responders compared to non-responders, during and after nCRT. This has been demonstrated in a recent meta-analysis on DWI for the detection of pCR in esophageal cancer patients who underwent nCRT followed by surgery (34). During the second to third week of nCRT, ADC was on average 26% higher in patients with TRG1 compared to patients with TRG2–4, as calculated in a random effects model (95% CI: 19–32%, P=0.60). Similarly, ADC was on average 34% higher in patients with TRG1 compared to patients with TRG2–4 at 3–9 weeks after completion of nCRT (95% CI: 12–55%, P=0.53).
A prospective multicenter study, that has been published after the above mentioned meta-analysis, had similar findings in a cohort of 69 patients (35). At approximately 2 weeks after start of nCRT, mean ADC values compared to start of nCRT (ΔADCmean) had increased more in patients with TRG1 [median ΔADCmean +28%, interquartile range (IQR): 15–39%] than in patients with TRG2–4 (median ΔADCmean +11%, IQR: 4–17%). At 5 weeks after completion of nCRT, ADC values had also more pronouncedly increased in patients with TRG1 (ΔADCmean +34%, IQR: 13–46%) than in patients with TRG 2–4 (ΔADCmean +20%, IQR: 10–38%), but this result was not statistically significant.
Moreover, in a study on 22 patients who underwent nCRT, sensitivity for detection of tumor (ypT) improved by addition of T2 MRI assessment to bite-on-bite biopsies (36). Sensitivity was 89% with MRI plus bite-on-bite biopsies, compared to a sensitivity of 33% with bite-on-bite biopsies only. However, this improvement was at the cost of specificity (50% specificity with MRI plus bite-on-bite biopsies versus 100% with bite-on-bite biopsies only). MRI did not improve the detection of lymph nodes that were detected with EUS-FNA, which was explained by a limited field of view on the MRI scan in this study.
MRI is not yet routinely used in CREs after nCRT. A prospective diagnostic study is being conducted that investigates the prediction of pCR using DWI and DCE-MRI, 18F-FDG PET/CT and circulating tumor DNA (ctDNA) obtained before, during and after nCRT (37). This study will hopefully provide new insights in the multimodal diagnostic assessment of residual tumor after nCRT.
18F-FDG PET/MRI
MRI can be used as a single modality or can be fully integrated with PET in a PET/MRI system, which is a recently developed technique. Possibly, 18F-FDG PET/MRI imaging provides additional anatomical and functional value over 18F-FDG PET correlated to (low dose) CT, for example by discrimination of post-radiation inflammation from residual tumor after nCRT. Quantitative 18F-FDG PET and MRI parameters appear uncorrelated and have shown complementary value in the prediction of pCR after nCRT (35,38). Visualization of the esophagus with MRI is challenging, however, due to cardiorespiratory motion in the mediastinum. Mediastinal-specific scanning protocols are therefore developed for 18F-FDG PET/MRI to acquire high quality images of the esophagus (39).
Today, few studies are available that report the diagnostic value of 18F-FDG PET/MRI in esophageal cancer. In 2014, a study was published that compared sequential 18F-FDG PET/MRI with EUS, diagnostic CT and 18F-FDG PET/CT, showing overall good performance with 18F-FDG PET/MRI (40). Fifteen patients, who did not receive neoadjuvant treatment, underwent staging with these modalities within 2 weeks before surgery. 18F-FDG PET/CT was not included in analysis of pT-stage, since no intravenous contrast was administered to perform reliable assessment of the primary tumor. Accuracy for pT-stage was 67% with FDG-PET/MRI, versus 87% with EUS and 33% with diagnostic CT. 18F-FDG PET/MRI had highest accuracy of 83% for nodal staging, followed by an accuracy of 75% with EUS, 67% with 18F-FDG PET/CT and 50% with diagnostic CT (40).
In a feasibility study, integrated 18F-FDG PET/MRI was compared to 18F-FDG PET/CT in a series of 16 patients who underwent 18F-FDG PET/MRI immediately after 18F-FDG PET/CT (41). Assessments of the primary tumor, lymph nodes and distant metastases were compared between the modalities. Radiological T-stage tended to be overestimated on 18F-FDG PET/CT compared to 18F-FDG PET/MRI. This was demonstrated by an inter-agreement Cohen’s kappa of 0.33 for T-stage and a correlation coefficient of 0.64 for tumor wall thickness. The discrepancy in T-stage might be caused by better visualization of the esophageal wall layers and surrounding tissue on 18F-FDG PET/MRI. Good agreement was seen between the modalities for N- and M-staging (Cohen’s kappa’s >0.85). The pre-treatment radiological assessments in this study were not compared to the findings in the resection specimens. Therefore, the true diagnostic accuracy for pathologic staging with 18F-FDG PET/MRI is not known at present.
Based on the above-discussed studies, staging with 18F-FDG PET/MRI seems non-inferior to 18F-FDG PET/CT. Further studies should continue to evaluate the accuracy of pre- and post-nCRT radiological staging with 18F-FDG PET/MRI in comparison to histopathology in the resection specimen.
Novel PET tracers
Radionuclide tracers are radioactively labeled substances that can bind to a target of interest in the body. Radioactively labelled glucose with 18fluor (18F-FDG) is commonly used in diagnostic oncological imaging. However, the use of 18F-FDG as a tracer has limitations since 18F-FDG cannot discriminate glucose metabolism in cancerous tissue from glucose metabolism in other cells. Several tracers that aim for more selective cancer imaging compared to 18F-FDG have been investigated over the past years, but these have not (yet) entered clinical routine (42-44). The tracer 18F-3’-deoxy-3’-fluorothymidine (18F-FLT) has been developed to image cell proliferation (44). 18F-FLT is phosphorylated by thymidine kinase-1, an enzyme involved during cell proliferation. As a result, 18F-FLT is retained in dividing cells. 18F-FLT might therefore be a surrogate marker for thymidine kinase-1 activity and cell proliferation. At the same time, 18F-FLT accumulates intensely in the physiological bone marrow and the liver and is thus of limited use in assessment of these regions (44). To overcome this limitation, a filtering technique applied to 18F-FLT PET has been investigated to improve tumor-to-background visualization in a small study of ten patients with esophageal or gastric cancer and liver metastases (45). 18F-FLT PET seems to be inferior to 18F-FDG PET before neoadjuvant treatment, based on results of two feasibility studies with more false-negatives at baseline staging with 18F-FLT PET than with 18F-FDG PET (46,47). Nevertheless, 18F-FLT PET for (early) prediction of response to neoadjuvant treatment with 18F-FLT PET has been proposed, since 18F-FLT uptake seems not to be affected by inflammatory processes, unlike 18F-FDG (46,48). The usefulness of 18F-FLT PET/CT in esophageal cancer still needs to be confirmed in larger trials.
Fibroblast activation protein (FAP) has been recently proposed as a promising diagnostic and therapeutic target in several types of cancer, including esophageal cancer (49-51). FAP is a membrane-bound protease which is specifically found on the surface of diseased cells, such as on stromal fibroblasts in almost all epithelial carcinomas, on hepatic cells in liver fibrosis and on aortic smooth muscle cells in aortic plaques (52,53). The exact role of FAP-expressing cells in the tumor microenvironment is unknown. FAP is suggested to induce immunosuppressive pathways and to promote angiogenesis (53). FAP can be targeted with FAP-selective inhibitors (FAPI). Progress has been made to develop FAP-targeted therapy and to visualize FAP-expressing cells using PET. 68Gallium-labelled FAPI (68Ga-FAPI) tracers have been recently developed and tested in patients (49-51). This type of radiotracer has been shown to bind to cancer-associated fibroblasts in the stroma of various solid tumors and distant metastases (51). 68Ga-FAPI depicts a better tumor-to-background contrast than for example 18F-FDG, since no accumulation in healthy tissue is present and the tracer is rapidly cleared from the body. In six esophageal cancer patients, uptake of FAPI was more than six times higher in the primary tumor compared to the background activity, which is substantially higher than with 18F-FDG (49). Further research on pharmacokinetic characteristics of FAPI tracer derivatives is ongoing and is aimed at improving for example tumor retention time (50). Further clinical studies are required to investigate sensitivity and specificity of 68Ga-FAPI to detect histologically confirmed lesions in esophageal cancer.
Radiomics
Radiomics is being extensively studied to optimize the assessment of medical imaging. Radiomics is a high-throughput method to obtain quantitative imaging features representing tumor characteristics that may correlate with the underlying biology, most of which cannot be seen by the unaided eye (54). Radiomics can be performed using hand-crafted imaging features or through fully automated feature creation using deep neural networks (Figure 1). Hand-crafted radiomic features are extracted from the image by the application of pre-specified mathematical formulas representing shape, pixel intensity statistics, and texture metrics of a tumor. With fully automated radiomics, imaging features salient with respect to a defined outcome are automatically computed and selected through deep convolutional neural networks (CNNs), e.g., to detect abnormal lesions or to predict response (55). Fully automated radiomics is thus less time-consuming and independent on subjective human assessment. However, its use can be limited since deep learning requires large amounts of well-curated data, which is often lacking in healthcare centers. Models can be developed using radiomics for radiologic and nuclear scans, but also on real-time endoscopic imaging. Well-performing models can subsequently be incorporated into computer-aided detection (CAD) systems (56).
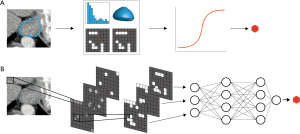
Many studies have developed a hand-crafted radiomic signature (i.e., combinations of weighted radiomic features) for prediction of response after nCRT in esophageal cancer patients. A diagnostic CT-based radiomic signature for prediction of pCR after nCRT yielded an area under the receiver operating characteristic curve (AUC) of 0.79 in the validation cohort of one study (57). The final prediction model included a shape feature (surface-to-volume ratio), a grey-level intensity feature and three textural features. A class of texture features in CT-scans called gray-level co-occurrence matrices (GLCM) was examined in another study of 36 patients (58). GLCM features before neoadjuvant chemo(radio)therapy were significantly different in adenocarcinoma patients with ypT0–T2 versus ypT3–T4 tumors. This difference was not seen in squamous cell carcinoma patients. In another retrospective study, CT-radiomic features were found to have prognostic value (59). The radiomic model predicted 3-year overall survival with an AUC of 0.61 in a validation cohort of patients treated with CROSS followed by surgery.
A considerable number of 18F-FDG PET studies have been performed that showed value of hand-crafted radiomics to predict treatment response after nCRT according to RECIST (Response Evaluation Criteria In Solid Tumors) or histopathologic assessment. Some studies showed that radiomic features had better predictive performance than conventional standardized uptake values on PET (60-72). Some studies also reported that the use of features from both pre- and post-nCRT scans yielded better predictive performance of pCR than features at one time-point (60,65,66,68,70). Furthermore, in two studies with mostly adenocarcinoma patients, prediction of TRG1 after neoadjuvant treatment had best performance with a model combining clinical parameters and radiomic PET features (60,68). In another cohort of solely squamous cell carcinoma patients, higher pre-treatment histogram entropy, a radiomic feature that represents the randomness of pixel values, was significantly associated with residual tumor after nCRT (72). The combination of radiomics with clinical variables in this study yielded an AUC of 0.82 in a small validation cohort of 16 patients.
MRI radiomic signatures have been developed on DW-MRI, 18F-FDG PET/MRI and T2 MRI scans to predict survival, synchronous metastases and metastatic lymph nodes in esophageal cancer patients with good results (73-75). Response assessment with MRI-based radiomics has been investigated in one study (76). Squamous cell carcinoma patients who obtained a pCR after nCRT had higher probability towards lower pre-treatment ADC values as shown by high skewness (i.e., a measure of asymmetry) and high kurtosis (i.e., a measure of tailedness) in histogram analysis. Further quantification of ADC values with radiomics on MRI scans might be valuable in response prediction.
In addition to hand-crafted radiomics, deep learning is also emerging for prediction of various outcomes. Artificial neural networks have been applied to classify CT- and MRI-based features to predict response to CRT according to RECIST criteria (77,78). A combination of deep learning and hand-crafted preoperative radiomic features could discriminate patients with metastatic lymph nodes from those without metastatic lymph nodes with good accuracy in an independent validation dataset (c-statistic of 0.84) (79). Also, deep-learned PET features had an AUC of 0.74 for a model that predicted death at 1 year after diagnosis in squamous cell carcinoma patients (80).
Despite the fact that many promising studies on radiomics have been published, it is often difficult to compare their outcomes and clinical usefulness. Comparison is not always possible when models have been developed on patients with different treatment regimens, or when different methods for response assessment have been conducted (e.g., RECIST versus histopathology). The methodology for feature extraction and feature selection influences study results (62). Different validation approaches can affect the final performance of the models. Apart from difficulties in the comparison of studies on radiomics, these studies can have limited reproducibility. Acquisition and reconstruction protocols differ across hospitals, which lead to slightly different radiomic feature values in different centers. Therefore, robustness studies are conducted to identify features that are independent of scanners or (timing of) intravenous contrast administration (81-84). Furthermore, research is ongoing to optimize the infrastructure for conducting studies with radiomics, for example through distributed learning (85). With distributed learning, anonymized imaging data can be used for model development without leaving the hospitals. This overcomes limitations of strict privacy regulations and will hopefully contribute to larger available datasets, enabling the development and validation of clinically useful radiomic models.
Novel biomarkers
Volatile organic compounds (VOCs)
VOCs are found in breath samples and have been described as novel biomarkers to detect various diseases, such as inflammatory bowel disease, asthma and colorectal cancer (86-88). VOCs are metabolites originating from cell processes and can diffuse to exhaled breath, but also to blood, saliva and urine (89). VOC profiles may be specific for biochemical processes in certain cancer types, reflecting for example genetic or protein changes in cancer cells, oxidative stress, or alterations in the microbiome (90). The field of VOC analysis is also referred to as “breathomics” and is attractive in terms of non-invasiveness and potential cost-effectiveness. VOCs in exhaled breath might have potential in the detection of esophagogastric cancer. A multicenter validation study tested a previously constructed model on VOCs in breath samples, that was analyzed with selected ion flow-tube mass spectrometry, a method to quantify the number and composition of VOCs (91). The model was tested on a cohort of 163 patients with esophageal or gastric cancer and 172 control patients with benign conditions such as hiatal hernia or esophagitis. Good performance for the detection of patients with cancer was reached with 80% sensitivity, 81% specificity and an AUC of 0.85.
Breath sample testing has also been studied in the detection of Barrett’s esophagus with an electronic nose device (eNose) (92,93). eNoses detect VOCs with chemical sensors in the device, which translate the activation signals into a breathprint. The data are consequently processed by machine learning algorithms to link patterns of VOCs to the disease of interest. An eNose was able to discriminate 129 patients with Barrett’s esophagus from 273 healthy controls and patients with gastro-esophageal reflux disease with a sensitivity of 91%, a specificity of 74% and an AUC of 0.91 (93). Moreover, the eNose could classify Barrett’s esophagus from gastro-esophageal reflux disease, albeit with a more moderate sensitivity of 64%, a specificity of 74% and an AUC of 0.73. These data indicate an ability of VOCs to discriminate various conditions in a screening-setting. Whether this also applies in a treatment setting for esophageal cancer needs to be determined in future studies.
Liquid biopsies
A liquid biopsy is an analysis on body fluids. A minimally-invasive liquid biopsy on a blood sample aims to detect circulating tumor cells (CTCs) or tumor components, which include ctDNA, tumor-derived exosomes, circulating tumor RNA and circulating tumor micro RNA. Liquid biopsies are of interest for developing targeted treatment in the pathway of developing metastases, but also for detection of residual disease and monitoring disease recurrence with repeated analyses (94,95).
In liquid biopsies, biomarker levels can be quantified and tumor-specific gene mutations can be characterized (96,97). These analyses can be limited due to low concentrations of CTCs in blood and heterogeneity in cell-surface antigens or tumor-specific gene mutations (98,99). Accurate assays are under development to assure reproducibility in different laboratories. This area of research is rapidly evolving (100,101).
Pre-treatment presence of CTCs has been associated with disease progression after chemo(radio)therapy and/or esophagectomy in squamous cell carcinoma (102,103). These findings are consistent with those of a prospective study on adenocarcinoma and squamous cell carcinoma patients that observed a correlation between baseline CTC-positivity and biopsy-proven tumor relapse after surgery (104). Levels of CTCs, as detected in 9 of 20 adenocarcinoma patients in another study, increased after neoadjuvant chemo(radio)therapy and decreased after surgery (99). The clinical impact of CTCs will be investigated by the same research group in ongoing side-studies of the prospective randomized controlled ESOPEC trial. The ESOPEC trial compares perioperative chemotherapy (FLOT) with nCRT (CROSS) (105).
Furthermore, a retrospective study showed that presence of ctDNA after chemoradiation correlated with tumor progression and development of distant metastases (106). Increasing ctDNA-levels preceded disease progression as observed with 18F-FDG PET/CT in some patients in that study. Similar findings have been observed in another study, albeit in a palliative setting (98). However, these studies included only few patients and results may have been influenced by selection bias. Results of prospective diagnostic trials will have to be awaited to assess the clinical value of ctDNA in prognostication or response assessment (9,37,105,107).
Conclusions
Tumor detection after nCRT, currently performed with endoscopy with bite-on-bite biopsies, EUS-FNA of suspicious lymph nodes and 18F-FDG PET/CT, might be further optimized. The combination of bite-on-bite biopsies and EUS-FNA has been shown to improve locoregional tumor detection as compared to regular biopsies with EUS-FNA. The sampling of larger mucosal areas within the initial tumor region, preferably in combination with deeper biopsies, is proposed to further reduce the rate of false-negative biopsies. Accuracy of EUS-FNA might be optimized by sampling all visible lymph nodes. Additional techniques to EUS, such as EUS elastography or CEH-EUS, might enhance assessment of suspicious lymph nodes, but require further research to assess clinical usefulness after nCRT. Serial 18F-FDG PET/CT scans appear valuable to monitor local tumor regrowth beyond 12 weeks after nCRT, when radiation-esophagitis has resolved. Instead of 18F-FDG PET/CT alone, imaging assessment could be supplemented with qualitative and quantitative MRI, or with integrated 18F-FDG PET/MRI. Other tracers than 18F-FDG, such as 68Ga-FAPI, are explored that allow for more specific cancer imaging. Further emerging fields of research are the application of non-invasive radiomics on standard-of-care medical imaging, the use of VOCs and the application of liquid biopsies for early and minimally-invasive tumor detection.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Sjoerd Lagarde, Bas Wijnhoven, and Florian Lordick) for the series “Novel Developments in the Multimodality Treatment of Esophageal Cancer” published in Annals of Esophagus. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at: http://dx.doi.org/10.21037/aoe-2020-02). The series “Novel Developments in the Multimodality Treatment of Esophageal Cancer” was commissioned by the editorial office without any funding or sponsorship. JJB van Lanschot serves as an unpaid editorial consultant of Annals of Esophagus from Nov 2018 to Oct 2020. HCW reports grants from Dutch Cancer Society (KWF) during the conduct of the study and owns minority shares from Oncoradiomics, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Allum WH, Stenning SP, Bancewicz J, et al. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol 2009;27:5062-7. [Crossref] [PubMed]
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Shapiro J, van Lanschot JJB, Hulshof M, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. [Crossref] [PubMed]
- Noordman BJ, Verdam MGE, Lagarde SM, et al. Impact of neoadjuvant chemoradiotherapy on health-related quality of life in long-term survivors of esophageal or junctional cancer: results from the randomized CROSS trial. Ann Oncol 2018;29:445-51. [Crossref] [PubMed]
- Clinicaltrials.gov. Comparison of systematic surgery versus surveillance and rescue surgery in operable oesophageal cancer with a complete clinical response to radiochemotherapy (Esostrate). Available online: https://clinicaltrials.gov/ct2/show/NCT02551458, accessed March 25, 2020.
- Noordman BJ, Wijnhoven BPL, Lagarde SM, et al. Neoadjuvant chemoradiotherapy plus surgery versus active surveillance for oesophageal cancer: a stepped-wedge cluster randomised trial. BMC Cancer 2018;18:142. [Crossref] [PubMed]
- Noordman BJ, Spaander MCW, Valkema R, et al. Detection of residual disease after neoadjuvant chemoradiotherapy for oesophageal cancer (preSANO): a prospective multicentre, diagnostic cohort study. Lancet Oncol 2018;19:965-74. [Crossref] [PubMed]
- Zhang X, Eyck BM, Yang Y, et al. Accuracy of detecting residual disease after neoadjuvant chemoradiotherapy for esophageal squamous cell carcinoma (preSINO trial): a prospective multicenter diagnostic cohort study. BMC Cancer 2020;20:194. [Crossref] [PubMed]
- Shapiro J, ten Kate FJ, van Hagen P, et al. Residual esophageal cancer after neoadjuvant chemoradiotherapy frequently involves the mucosa and submucosa. Ann Surg 2013;258:678-88; discussion 88-9. [Crossref] [PubMed]
- Chao YK, Chuang WY, Yeh CJ, et al. Anatomical distribution of residual cancer in patients with oesophageal squamous cell carcinoma who achieved clinically complete response after neoadjuvant chemoradiotherapy. Eur J Cardiothorac Surg 2018;53:201-8. [Crossref] [PubMed]
- Docimo S Jr, Al-Mansour M, Tsuda S. SAGES TAVAC safety and efficacy analysis WATS(3D) (CDx Diagnostics, Suffern, NY). Surg Endosc 2020;34:3743-7. [Crossref] [PubMed]
- Canto MI, Montgomery E. Wide-area transepithelial sampling with 3-dimensional cytology: Does it detect more dysplasia or yield more hype? Gastrointest Endosc 2018;87:356-9. [Crossref] [PubMed]
- Antonini F, Delconte G, Fuccio L, et al. EUS-guided tissue sampling with a 20-gauge core biopsy needle for the characterization of gastrointestinal subepithelial lesions: a multicenter study. Endosc Ultrasound 2019;8:105-10. [Crossref] [PubMed]
- Hirata N, Kawamoto K, Ueyama T, et al. Using endosonography to assess the effects of neoadjuvant therapy in patients with advanced esophageal cancer. AJR Am J Roentgenol 1997;169:485-91. [Crossref] [PubMed]
- Isenberg G, Chak A, Canto MI, et al. Endoscopic ultrasound in restaging of esophageal cancer after neoadjuvant chemoradiation. Gastrointest Endosc 1998;48:158-63. [Crossref] [PubMed]
- Willis J, Cooper GS, Isenberg G, et al. Correlation of EUS measurement with pathologic assessment of neoadjuvant therapy response in esophageal carcinoma. Gastrointest Endosc 2002;55:655-61. [Crossref] [PubMed]
- Jost C, Binek J, Schuller JC, et al. Endosonographic radial tumor thickness after neoadjuvant chemoradiation therapy to predict response and survival in patients with locally advanced esophageal cancer: a prospective multicenter phase ll study by the Swiss Group for Clinical Cancer Research (SAKK 75/02). Gastrointest Endosc 2010;71:1114-21. [Crossref] [PubMed]
- van der Bogt RD, Noordman BJ, Krishnadath KK, et al. Endoscopic ultrasound measurements for detection of residual disease after neoadjuvant chemoradiotherapy for esophageal cancer. Endoscopy 2019;51:326-32. [Crossref] [PubMed]
- van der Bogt RD, van der Wilk BJ, Poley JW, et al. Endoscopic ultrasound and fine-needle aspiration for the detection of residual nodal disease after neoadjuvant chemoradiotherapy for esophageal cancer. Endoscopy 2020;52:186-92. [Crossref] [PubMed]
- Colaiacovo R, Costa ADS Jr, Paulo GA, et al. Echoendoscopy with elastography in mediastinal lymph nodes. Einstein (Sao Paulo) 2019;17:eMD5157. [Crossref] [PubMed]
- Knabe M, Günter E, Ell C, et al. Can EUS elastography improve lymph node staging in esophageal cancer? Surg Endosc 2013;27:1196-202. [Crossref] [PubMed]
- Sazuka T, Akai T, Uesato M, et al. Assessment for diagnosis of lymph node metastasis in esophageal cancer using endoscopic ultrasound elastography. Esophagus 2016;13:254-63. [Crossref] [PubMed]
- Paterson S, Duthie F, Stanley AJ. Endoscopic ultrasound-guided elastography in the nodal staging of oesophageal cancer. World J Gastroenterol 2012;18:889-95. [Crossref] [PubMed]
- Kannengiesser K, Mahlke R, Petersen F, et al. Instant evaluation of contrast enhanced endoscopic ultrasound helps to differentiate various solid pancreatic lesions in daily routine. World J Clin Cases 2019;7:19-27. [Crossref] [PubMed]
- Eyck BM, Onstenk BD, Noordman BJ, et al. Accuracy of detecting residual disease after neoadjuvant chemoradiotherapy for esophageal Cancer: a systematic review and meta-analysis. Ann Surg 2020;271:245-56. [Crossref] [PubMed]
- Valkema MJ, Noordman BJ, Wijnhoven BPL, et al. Accuracy of (18)F-FDG PET/CT in predicting residual disease after neoadjuvant chemoradiotherapy for esophageal cancer. J Nucl Med 2019;60:1553-9. [Crossref] [PubMed]
- Wei Y, Wu S, Gao F, et al. Esophageal carcinoma: Ex vivo evaluation by high-spatial-resolution T2 -mapping MRI compared with histopathological findings at 3.0T. J Magn Reson Imaging 2017;45:1609-16. [Crossref] [PubMed]
- Riddell AM, Hillier J, Brown G, et al. Potential of surface-coil MRI for staging of esophageal cancer. AJR Am J Roentgenol 2006;187:1280-7. [Crossref] [PubMed]
- Dirix P, Haustermans K, Vandecaveye V. The value of magnetic resonance imaging for radiotherapy planning. Semin Radiat Oncol 2014;24:151-9. [Crossref] [PubMed]
- Koh DM, Padhani AR. Diffusion-weighted MRI: a new functional clinical technique for tumour imaging. Br J Radiol. 2006;79:633-5. [Crossref] [PubMed]
- Chang EY, Li X, Jerosch-Herold M, et al. The evaluation of esophageal adenocarcinoma using dynamic contrast-enhanced magnetic resonance imaging. J Gastrointest Surg 2008;12:166-75. [Crossref] [PubMed]
- Heethuis SE, Goense L, van Rossum PSN, et al. DW-MRI and DCE-MRI are of complementary value in predicting pathologic response to neoadjuvant chemoradiotherapy for esophageal cancer. Acta Oncol 2018;57:1201-8. [Crossref] [PubMed]
- Maffazzioli L, Zilio MB, Klamt AL, et al. ADC as a predictor of pathologic response to neoadjuvant therapy in esophageal cancer: a systematic review and meta-analysis. Eur Radiol 2020;30:3934-42. [Crossref] [PubMed]
- Borggreve AS, Goense L, van Rossum PSN, et al. Preoperative prediction of pathologic response to neoadjuvant chemoradiotherapy in patients with esophageal cancer using (18)F-FDG PET/CT and DW-MRI: a prospective multicenter study. Int J Radiat Oncol Biol Phys 2020;106:998-1009. [Crossref] [PubMed]
- Vollenbrock SE, van Dieren JM, Voncken FEM, et al. Added value of MRI to endoscopic and endosonographic response assessment after neoadjuvant chemoradiotherapy in oesophageal cancer. Eur Radiol 2020;30:2425-34. [Crossref] [PubMed]
- Borggreve AS, Mook S, Verheij M, et al. Preoperative image-guided identification of response to neoadjuvant chemoradiotherapy in esophageal cancer (PRIDE): a multicenter observational study. BMC Cancer 2018;18:1006. [Crossref] [PubMed]
- Goense L, Heethuis SE, van Rossum PSN, et al. Correlation between functional imaging markers derived from diffusion-weighted MRI and 18F-FDG PET/CT in esophageal cancer. Nucl Med Commun 2018;39:60-7. [Crossref] [PubMed]
- Peerlings J, Paulis L, Mitea C, et al. Performing clinical 18F-FDG-PET/MRI of the mediastinum optimising a dedicated, patient-friendly protocol. Nucl Med Commun 2019;40:815-26. [Crossref] [PubMed]
- Lee G, I H, Kim SJ, et al. Clinical implication of PET/MR imaging in preoperative esophageal cancer staging: comparison with PET/CT, endoscopic ultrasonography, and CT. J Nucl Med 2014;55:1242-7. [Crossref] [PubMed]
- Linder G, Korsavidou-Hult N, Bjerner T, et al. (18)F-FDG-PET/MRI in preoperative staging of oesophageal and gastroesophageal junctional cancer. Clin Radiol 2019;74:718-25. [Crossref] [PubMed]
- Jager PL, Que TH, Vaalburg W, et al. Carbon-11 choline or FDG-PET for staging of oesophageal cancer? Eur J Nucl Med 2001;28:1845-9. [Crossref] [PubMed]
- Dong Y, Wei Y, Chen G, et al. Relationship between clinicopathological characteristics and PET/CT uptake in esophageal squamous cell carcinoma: [(18)F]alfatide versus [(18)F]FDG. Mol Imaging Biol 2019;21(1:175-82.
- Shields AF, Grierson JR, Dohmen BM, et al. Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nat Med 1998;4:1334-6. [Crossref] [PubMed]
- Sharma R, Mapelli P, Hanna GB, et al. Evaluation of (18)F-fluorothymidine positron emission tomography ([(18)F]FLT-PET/CT) methodology in assessing early response to chemotherapy in patients with gastro-oesophageal cancer. EJNMMI Res 2016;6:81. [Crossref] [PubMed]
- Park SH, Ryu JS, Oh SJ, et al. The feasibility of (18)F-fluorothymidine PET for prediction of tumor response after induction chemotherapy followed by chemoradiotherapy with S-1/oxaliplatin in patients with resectable esophageal cancer. Nucl Med Mol Imaging 2012;46:57-64. [Crossref] [PubMed]
- van Westreenen HL, Cobben DC, Jager PL, et al. Comparison of 18F-FLT PET and 18F-FDG PET in esophageal cancer. J Nucl Med 2005;46:400-4. [PubMed]
- Gerbaudo VH, Killoran JH, Kim CK, et al. Pilot study of serial FLT and FDG-PET/CT imaging to monitor response to neoadjuvant chemoradiotherapy of esophageal adenocarcinoma: correlation with histopathologic response. Ann Nucl Med 2018;32:165-74. [Crossref] [PubMed]
- Kratochwil C, Flechsig P, Lindner T, et al. (68)Ga-FAPI PET/CT: tracer uptake in 28 different kinds of cancer. J Nucl Med 2019;60:801-5. [Crossref] [PubMed]
- Loktev A, Lindner T, Burger EM, et al. Development of fibroblast activation protein-targeted radiotracers with improved tumor retention. J Nucl Med 2019;60:1421-9. [Crossref] [PubMed]
- Loktev A, Lindner T, Mier W, et al. A tumor-imaging method targeting cancer-associated fibroblasts. J Nucl Med 2018;59:1423-9. [Crossref] [PubMed]
- Brokopp CE, Schoenauer R, Richards P, et al. Fibroblast activation protein is induced by inflammation and degrades type I collagen in thin-cap fibroatheromata. Eur Heart J 2011;32:2713-22. [Crossref] [PubMed]
- Hamson EJ, Keane FM, Tholen S, et al. Understanding fibroblast activation protein (FAP): substrates, activities, expression and targeting for cancer therapy. Proteomics Clin Appl 2014;8:454-63. [Crossref] [PubMed]
- Lambin P, Leijenaar RTH, Deist TM, et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol 2017;14:749-62. [Crossref] [PubMed]
- Horie Y, Yoshio T, Aoyama K, et al. Diagnostic outcomes of esophageal cancer by artificial intelligence using convolutional neural networks. Gastrointest Endosc 2019;89:25-32. [Crossref] [PubMed]
- de Groof AJ, Struyvenberg MR, van der Putten J, et al. Deep-learning system detects neoplasia in patients with Barrett's esophagus with higher accuracy than endoscopists in a multistep training and validation study with benchmarking. Gastroenterology 2020;158:915-29.e4. [Crossref] [PubMed]
- Yang Z, He B, Zhuang X, et al. CT-based radiomic signatures for prediction of pathologic complete response in esophageal squamous cell carcinoma after neoadjuvant chemoradiotherapy. J Radiat Res 2019;60:538-45. [Crossref] [PubMed]
- Zhang YH, Herlin G, Rouvelas I, et al. Texture analysis of computed tomography data using morphologic and metabolic delineation of esophageal cancer-relation to tumor type and neoadjuvant therapy response. Dis Esophagus 2019;32:doy096. [Crossref] [PubMed]
- Larue RT, Klaassen R, Jochems A, et al. Pre-treatment CT radiomics to predict 3-year overall survival following chemoradiotherapy of esophageal cancer. Acta Oncol 2018;57:1475-81. [Crossref] [PubMed]
- Beukinga RJ, Hulshoff JB, Mul VEM, et al. Prediction of response to neoadjuvant chemotherapy and radiation therapy with baseline and restaging (18)F-FDG PET imaging biomarkers in patients with esophageal cancer. Radiology 2018;287:983-92. [Crossref] [PubMed]
- Beukinga RJ, Hulshoff JB, van Dijk LV, et al. Predicting response to neoadjuvant chemoradiotherapy in esophageal cancer with textural features derived from pretreatment (18)F-FDG PET/CT imaging. J Nucl Med 2017;58:723-9. [Crossref] [PubMed]
- Desbordes P, Ruan S, Modzelewski R, et al. Predictive value of initial FDG-PET features for treatment response and survival in esophageal cancer patients treated with chemo-radiation therapy using a random forest classifier. PLoS One 2017;12:e0173208. [Crossref] [PubMed]
- Foley KG, Hills RK, Berthon B, et al. Development and validation of a prognostic model incorporating texture analysis derived from standardised segmentation of PET in patients with oesophageal cancer. Eur Radiol 2018;28:428-36. [Crossref] [PubMed]
- Nakajo M, Jinguji M, Nakabeppu Y, et al. Texture analysis of (18)F-FDG PET/CT to predict tumour response and prognosis of patients with esophageal cancer treated by chemoradiotherapy. Eur J Nucl Med Mol Imaging 2017;44:206-14. [Crossref] [PubMed]
- Tan S, Kligerman S, Chen W, et al. Spatial-temporal [18F]FDG-PET features for predicting pathologic response of esophageal cancer to neoadjuvant chemoradiation therapy. Int J Radiat Oncol Biol Phys 2013;85:1375-82. [Crossref] [PubMed]
- Tan S, Zhang H, Zhang Y, et al. Predicting pathologic tumor response to chemoradiotherapy with histogram distances characterizing longitudinal changes in 18F-FDG uptake patterns. Med Phys 2013;40:101707. [Crossref] [PubMed]
- Tixier F, Le Rest CC, Hatt M, et al. Intratumor heterogeneity characterized by textural features on baseline 18F-FDG PET images predicts response to concomitant radiochemotherapy in esophageal cancer. J Nucl Med 2011;52:369-78. [Crossref] [PubMed]
- van Rossum PS, Fried DV, Zhang L, et al. The incremental value of subjective and quantitative assessment of 18F-FDG PET for the prediction of pathologic complete response to preoperative chemoradiotherapy in esophageal cancer. J Nucl Med 2016;57:691-700. [Crossref] [PubMed]
- Xiong J, Yu W, Ma J, et al. The role of PET-based radiomic features in predicting local control of esophageal cancer treated with concurrent chemoradiotherapy. Sci Rep 2018;8:9902. [Crossref] [PubMed]
- Yip SS, Coroller TP, Sanford NN, et al. Relationship between the temporal changes in positron-emission-tomography-imaging-based textural features and pathologic response and survival in esophageal cancer patients. Front Oncol 2016;6:72. [Crossref] [PubMed]
- Zhang H, Tan S, Chen W, et al. Modeling pathologic response of esophageal cancer to chemoradiation therapy using spatial-temporal 18F-FDG PET features, clinical parameters, and demographics. Int J Radiat Oncol Biol Phys 2014;88:195-203. [Crossref] [PubMed]
- Chen YH, Lue KH, Chu SC, et al. Combining the radiomic features and traditional parameters of (18)F-FDG PET with clinical profiles to improve prognostic stratification in patients with esophageal squamous cell carcinoma treated with neoadjuvant chemoradiotherapy and surgery. Ann Nucl Med 2019;33:657-70. [Crossref] [PubMed]
- Baiocco S, Sah BR, Mallia A, et al. Exploratory radiomic features from integrated (18)F-fluorodeoxyglucose positron emission tomography/magnetic resonance imaging are associated with contemporaneous metastases in oesophageal/gastroesophageal cancer. Eur J Nucl Med Mol Imaging 2019;46:1478-84. [Crossref] [PubMed]
- Li Z, Han C, Wang L, et al. Prognostic value of texture analysis based on pretreatment DWI-weighted MRI for esophageal squamous cell carcinoma patients treated with concurrent chemo-radiotherapy. Front Oncol 2019;9:1057. [Crossref] [PubMed]
- Qu J, Shen C, Qin J, et al. The MR radiomic signature can predict preoperative lymph node metastasis in patients with esophageal cancer. Eur Radiol 2019;29:906-14. [Crossref] [PubMed]
- Hirata A, Hayano K, Ohira G, et al. Volumetric histogram analysis of apparent diffusion coefficient for predicting pathological complete response and survival in esophageal cancer patients treated with chemoradiotherapy. Am J Surg 2020;219:1024-9. [Crossref] [PubMed]
- Hou Z, Li S, Ren W, et al. Radiomic analysis in T2W and SPAIR T2W MRI: predict treatment response to chemoradiotherapy in esophageal squamous cell carcinoma. J Thorac Dis 2018;10:2256-67. [Crossref] [PubMed]
- Hou Z, Ren W, Li S, et al. Radiomic analysis in contrast-enhanced CT: predict treatment response to chemoradiotherapy in esophageal carcinoma. Oncotarget 2017;8:104444-54. [Crossref] [PubMed]
- Wu L, Yang X, Cao W, et al. Multiple level CT radiomics features preoperatively predict lymph node metastasis in esophageal cancer: a multicentre retrospective study. Front Oncol 2020;9:1548. [Crossref] [PubMed]
- Yang CK, Yeh JC, Yu WH, et al. Deep convolutional neural network-based positron emission tomography analysis predicts esophageal cancer outcome. J Clin Med 2019;8:844. [Crossref] [PubMed]
- Larue RT, van Timmeren JE, de Jong EEC, et al. Influence of gray level discretization on radiomic feature stability for different CT scanners, tube currents and slice thicknesses: a comprehensive phantom study. Acta Oncol 2017;56:1544-53. [Crossref] [PubMed]
- Piazzese C, Foley K, Whybra P, et al. Discovery of stable and prognostic CT-based radiomic features independent of contrast administration and dimensionality in oesophageal cancer. PLoS One 2019;14:e0225550. [Crossref] [PubMed]
- Whybra P, Parkinson C, Foley K, et al. Assessing radiomic feature robustness to interpolation in (18)F-FDG PET imaging. Sci Rep 2019;9:9649. [Crossref] [PubMed]
- Peerlings J, Woodruff HC, Winfield JM, et al. Stability of radiomics features in apparent diffusion coefficient maps from a multi-centre test-retest trial. Sci Rep 2019;9:4800. [Crossref] [PubMed]
- Zerka F, Barakat S, Walsh S, et al. Systematic review of privacy-preserving distributed machine learning from federated databases in health care. JCO Clin Cancer Inform 2020;4:184-200. [Crossref] [PubMed]
- van Keulen KE, Jansen ME, Schrauwen RWM, et al. Volatile organic compounds in breath can serve as a non-invasive diagnostic biomarker for the detection of advanced adenomas and colorectal cancer. Aliment Pharmacol Ther 2020;51:334-46. [Crossref] [PubMed]
- Tiele A, Wicaksono A, Kansara J, et al. Breath analysis using eNose and ion mobility technology to diagnose inflammatory bowel disease-a pilot study. Biosensors (Basel) 2019;9:55. [Crossref] [PubMed]
- Azim A, Barber C, Dennison P, et al. Exhaled volatile organic compounds in adult asthma: a systematic review. Eur Respir J 2019;54:1900056. [Crossref] [PubMed]
- Chandrapalan S, Arasaradnam RP. Urine as a biological modality for colorectal cancer detection. Expert Rev Mol Diagn 2020;20:489-96. [Crossref] [PubMed]
- Haick H, Broza YY, Mochalski P, et al. Assessment, origin, and implementation of breath volatile cancer markers. Chem Soc Rev 2014;43:1423-49. [Crossref] [PubMed]
- Markar SR, Wiggins T, Antonowicz S, et al. Assessment of a noninvasive exhaled breath test for the diagnosis of oesophagogastric cancer. JAMA Oncol 2018;4:970-6. [Crossref] [PubMed]
- Chan DK, Zakko L, Visrodia KH, et al. Breath testing for Barrett's esophagus using exhaled volatile organic compound profiling with an electronic nose device. Gastroenterology 2017;152:24-6. [Crossref] [PubMed]
- Peters Y, Schrauwen RWM, Tan AC, et al. Detection of Barrett's oesophagus through exhaled breath using an electronic nose device. Gut 2020;69:1169-72. [Crossref] [PubMed]
- Follain G, Herrmann D, Harlepp S, et al. Fluids and their mechanics in tumour transit: shaping metastasis. Nat Rev Cancer 2020;20:107-24. [Crossref] [PubMed]
- Pantel K, Alix-Panabieres C. Tumour microenvironment: informing on minimal residual disease in solid tumours. Nat Rev Clin Oncol 2017;14:325-6. [Crossref] [PubMed]
- Mader S, Pantel K. Liquid biopsy: current status and future perspectives. Oncol Res Treat 2017;40:404-8. [Crossref] [PubMed]
- Matsuoka T, Yashiro M. Precision medicine for gastrointestinal cancer: recent progress and future perspective. World J Gastrointest Oncol 2020;12:1-20. [Crossref] [PubMed]
- Egyud M, Tejani M, Pennathur A, et al. Detection of circulating tumor DNA in plasma: a potential biomarker for esophageal ddenocarcinoma. Ann Thorac Surg 2019;108:343-9. [Crossref] [PubMed]
- Kuvendjiska J, Bronsert P, Martini V, et al. Non-metastatic esophageal adenocarcinoma: circulating tumor cells in the course of multimodal tumor treatment. Cancers (Basel) 2019;11:397. [Crossref] [PubMed]
- Woestemeier A, Harms-Effenberger K, Karstens KF, et al. Clinical relevance of circulating tumor cells in esophageal cancer detected by a combined MACS enrichment method. Cancers (Basel) 2020;12:718. [Crossref] [PubMed]
- Zhao A, Guo L, Xu J, et al. Identification and validation of circulating exosomes-based liquid biopsy for esophageal cancer. Cancer Med 2019;8:3566-74. [Crossref] [PubMed]
- Tanaka K, Yano M, Motoori M, et al. CEA-antigen and SCC-antigen mRNA expression in peripheral blood predict hematogenous recurrence after resection in patients with esophageal cancer. Ann Surg Oncol 2010;17:2779-86. [Crossref] [PubMed]
- Matsushita D, Uenosono Y, Arigami T, et al. Clinical significance of circulating tumor cells in peripheral blood of patients with esophageal squamous cell carcinoma. Ann Surg Oncol 2015;22:3674-80. [Crossref] [PubMed]
- Konczalla L, Ghadban T, Effenberger KE, et al. Prospective comparison of the prognostic relevance of circulating tumor cells in blood and disseminated tumor cells in bone marrow of a single patient's cohort with esophageal cancer. Ann Surg 2021;273:299-305. [PubMed]
- Hoeppner J, Lordick F, Brunner T, et al. ESOPEC: prospective randomized controlled multicenter phase III trial comparing perioperative chemotherapy (FLOT protocol) to neoadjuvant chemoradiation (CROSS protocol) in patients with adenocarcinoma of the esophagus (NCT02509286). BMC Cancer 2016;16:503. [Crossref] [PubMed]
- Azad TD, Chaudhuri AA, Fang P, et al. Circulating tumor DNA analysis for detection of minimal residual disease after chemoradiotherapy for localized esophageal cancer. Gastroenterology 2020;158:494-505.e6. [Crossref] [PubMed]
- Clinicaltrials.gov. Induction FLOT with CROSS CRT for esophageal cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT04028167, accessed April 24, 2020.
Cite this article as: Valkema MJ, Doukas M, Spaander MCW, Valkema R, Woodruff HC, van Lanschot JJB. Optimization of detection of residual disease after neoadjuvant therapy in patients with esophageal cancer. Ann Esophagus 2021;4:6.


