Imaging in evaluation of response to neo-adjuvant treatment
Introduction
Adenocarcinoma of the gastroesophageal junction (GEJ) is defined as a tumor which topographic center is within 5 cm proximal (or distal) to the anatomical cardia (1). Patients’ prognosis after upfront surgery is poor, due to high rates of complications, systemic and/or local recurrences (2). Multicenter, randomized trials [CROSS trial (3,4), POET trial (5)] have demonstrated benefit in terms of overall survival (OS) and/or progression free survival (PFS) in patients with locally advanced GEJ adenocarcinoma treated with neoadjuvant chemoradiotherapy (nCRT). In particular, the CROSS trial (3,4) demonstrated that preoperative administration of nCRT doubled the median overall survival of locally advanced esophageal and GEJ neoplasms in comparison to surgery alone and that 29% of these patients had a complete pathological response, suggesting that a subgroup of patients did not benefit from surgery, also considering its known side effects. Conversely, 18% of patients who underwent nCRT were deemed as being non-responders and did not benefit from nCRT but only suffered its side effects.
The proper assessment of tumor response after nCRT is therefore fundamental, but early definition of responder or non-responder status during (or even before) neoadjuvant therapy is even more important since it could enable tailored therapeutic plans, avoiding unnecessary treatment efforts and related adverse effects, with a major impact on patients’ quality of life as well as health care costs.
Although histopathology remains the gold standard for evaluation of response to nCRT, in some cases intermediate biopsies do not always predict outcome. In the diagnostic cohort preSANO trial (6), TRG3/4 neoplasms were missed in eight out of 26 cases with endoscopy guided biopsies and fine needle aspiration (FNA) and in four out of 41 cases with bite on bite biopsies and FNA performed four to six weeks after nCRT completion. In addition to invasiveness, bioptic procedures also have the limitation not to provide a reliable depiction of the entire tumor heterogeneity.
There is indeed an urgent need to identify a non-invasive tool able to depict the tumor microenvironment as a whole in this clinical setting. Morphologic cross-sectional imaging can here play a lead role: according to Response Evaluation Criteria in Solid Tumours (RECIST) version 1.1 (7), computed tomography (CT) has proven to be the most standardized, validated tool for tumor response assessment, based on dimensional comparison. However, its use may be limited especially when dealing with tumors with blurred contours and a consistent amount of fibrosis following nCRT, common feature in GEJ neoplasms (7). Additionally, it may become challenging to distinguish viable tumor from necrotic scar tissue (8), further increased by a clinically relevant delay between cell death and tumor shrinkage. The guideline endorsed imaging has therefore only limited role in assessing the actual benefit of nCRT in GEJ (7).
Functional imaging, such as positron emission tomography (PET), has also been evaluated as an alternative tool for evaluating tumor response, as it relies on a metabolic rather than a purely dimensional evaluation. An additional readily available imaging modality, magnetic resonance imaging (MRI) could allow collection of both morphologic and functional data (9), and act as a biomarker extraction tool. Unfortunately, in the past its broad use in the gastroesophageal tract has been precluded by technical difficulties.
Do solutions exist? In this article, the authors are providing data to support a proper choice of imaging techniques, including CT, MRI and PET, in this diagnostic dilemma. Examples of standard of care clinical practice, novel, quantitative diagnostic approaches, with a particular focus on radiomics, as well as the combination of different imaging modalities have been also reported
Methods
To evaluate the ability of the different available imaging techniques as non-invasive markers of treatment response, a literature review was conducted in a single publicly available database, MEDLINE (via PubMed). Two of the authors (VN, a third-year radiology resident, and PM, an experienced nuclear medicine physician) independently performed a computer-aided search for original articles not limited in the past, closed on March 1, 2020.
Study eligibility criteria
The review was based on PICOS (P: population, I: intervention, C: comparator, O: outcomes and S: study design) criteria. The population criteria were adults with GEJ adenocarcinoma, treated with nCRT or nCxT presenting with an imaging prior to and post treatment (and/or during treatment) and histology (intervention and comparator used as gold standard, respectively), regardless of stage and study design. A combination of the following words was used: “gastroesophageal junction or esophagogastric junction or esophageal or esophagus” and “adenocarcinoma or cancer or tumor or neoplasm” and “neoadjuvant therapy or neoadjuvant chemoradiation therapy” and “response assessment or prediction or early response” and one of the following “MRI or PET or PET/CT or PET/MR or PET/MRI or FDG PET/CT or FDG PET/MR or FDG PET/MRI (using slash or hyphen) or radiomics”. Abstracts, case reports and case series, editorials, letters to editor, animal studies and articles not in English language were excluded.
Selection of literature
After removing non-pertinent articles and duplicates, reviewers read the abstracts for eligibility based on reviewers read the abstracts for eligibility based on (I) studies in patients with locally advanced GEJ adenocarcinoma, (II) presence of imaging assessment before and after nCxT or nCRT (with or without intermediate imaging evaluation) and (III) reporting comparison of imaging findings to histopathology on surgically resected specimen or ultrasound guided biopsy. Histopathology was considered the reference standard for the purpose of this review. The reference lists of all selected articles were searched to identify further relevant studies and all studies selected were in adults regardless of prognosis and disease progression.
Results
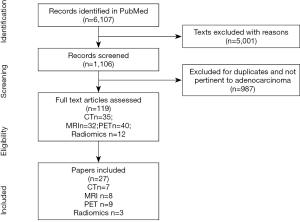
The literature search resulted in a total of 6,107. After initial exclusions and screening (see Figure 1), 119 were considered eligible and 27 included in this review. Results are presented based on the different imaging modalities and on the radiomics approach. After removing non-pertinent records, exclusions and duplicates, 119 articles were found eligible. Results are presented based on the different imaging modalities and those evaluating radiomic features. In particular, seven papers met the inclusion criteria for CT, 8 for MRI, 9 for PET and 3 for radiomics.
CT
A major limitation of literature concerning the role of contrast enhanced CT in determining GEJ tumours response after nCRT/nCxT was that the majority of the studies meeting inclusion criteria considers at once both GEJ and oesophageal/gastric neoplasms, irrespective of different management strategies and prognosis.
Although changes in CT tumour volume demonstrated greater correlation with pathological response than changes in tumour diameter and were associated with lower interobserver variability (10), it is more technically demanding and only a modest predictor of pathological response at best (10-12). The cut offs values used for changes in tumour volume to differentiate responders from non-responders ranged from 10% to 20% (10,13). Some studies (11,13) did not find a significant correlation between CT volumetric changes and pathological response, with a non-optimal performance assessed by ROC (receiver operating characteristic) curves of 0.63 [95% confidence interval (95% CI), 0.45–0.82] (13).
CT showed a sensitivity ranging from 33% to 55% and a specificity from 50% to 71% (14) in predicting pathological response and TNM stage after nCRT. Many factors may influence such a low accuracy, the most important being that CT, is unable to adequately help differentiate between T1, T2 and T3 disease (14,15) due to a poor contrast resolution, thus downstaging the assessment. Perfusion techniques have shown to be useful in identifying histopathological responders which are reported to have a lower tumor permeability in comparison to non-responders (16). Radiation therapy, on the other hand, may induce the release of pro angiogenic factors and stimulate angiogenesis, thus impairing an optimal perfusion evaluation (14,15).
Early response assessment is heavily hampered by inflammation and tissue edema occurring during nCRT (14,15). Van Heijl et al. (13) observed a paradoxical increase of CT median tumor volume measured between baseline and 14 days after the beginning of nCRT in histopathological responders as well as in non-responders. Discordant results were observed in a cohort of 31 patients with locally advanced GEJ neoplasms who underwent contrast enhanced CT scan before and two weeks after the beginning of nCxT (10), where early changes in CT tumor volume well predicted histopathological tumor response (sensitivity: 100%; specificity: 53%). In a large multicenter study evaluating combined endoscopy and CT (17), a good agreement was assessed between non-responders and histopathological response, with a very high negative predictive value (85–92%), even at interim assessment.
In another study using a CT perfusion scan, Lundsgaard Hansen et al. (16) reported a positive, significant correlation between an early decrease (after once cycle) in tumor permeability and overall clinical response after nCxT, based on dimensional criteria cut-off.
To our knowledge, no study specifically addresses the accuracy of CT in assessment of lymph node response after nCRT. In a cohort of 18 patients with esophageal or Siewert 1 GEJ neoplasms treated with either nCRT or nCxT, Giganti et al. (15) found that CT, relying on dimensional criteria alone, has low sensitivity and specificity (75% and 57%, respectively) in predicting N stage.
The selected studies assessing the role of CT in neoadjuvant treatment response in GEJ cancer are reported in Table 1.
Table 1
| Study | Localization | No. patients | Histology | Neoadjuvant therapy | Timing of scans (post treatment) | Pathological assessment | CT parameters | Results |
|---|---|---|---|---|---|---|---|---|
| Early response | ||||||||
| Beer |
GEJ cancer (Siewert I-II) | 31 (21 with pathological assessment) | AC | CxT | • Baseline | Modified Mandard score [Becker ( |
• Maximum tumor diameter | • Statistically significant differences in tumor volume and diameter (P=0.009 and P=0.011) delta baseline |
| • Interim (day 14-17) | • Grade 1 (0–10% residual tumor per tumor bed): histopathological R | • Semi automated tumor volume | • Statistically significant decrease in mean tumor volume |
|||||
| • Prior to surgical resection (day 17/21) | • Grade 2-3: NR | • Delta values of diameter and volume between among and between 3 time pointsA | • Post CRT (mean 73 days between scans) | |||||
| • Low interobserver variability for volumetric evaluations | ||||||||
| • Cut off −13.2% for diametric changes and 14.8% for volumetric changes in differentiating R from NR, with a sensitivity of 100% and a specificity of 53% for volumetric changes | ||||||||
| • Cut off −13.2% for diametric changes and 14.8% for volumetric changes in differentiating R from NR, with a sensitivity of 100% and a specificity of 53% for volumetric changes | ||||||||
| Van Heijl |
EC and GEJ cancer | 39 | AC; SCC | CRT | • Baseline | Mandard score ( |
3D volume | • Tumor volume increases in R and NR with relatively higher increase in the histopathological NR (22%), non-significantly different compared to NR (12%) |
| • Interim evaluation (day 15 during CRT) | • AUC for pathological response assessment using CT =0.63 (95% CI, 0.45–0.82) | |||||||
| • ΔCT volume cut-off >10%: sensitivity 19%, specificity 92%, PPV 83%, NPV 36% | ||||||||
| • ΔCT volume cut-off >20%: sensitivity 8%, specificity 100%, PPV 100%, NPV 35% | ||||||||
| Early and late response | ||||||||
| Lundsgaard Hansen |
Gastric and GEJ cancer | 28 (26 all scans) | AC | CxT | • Baseline | Mandard score: R defined; Grade 1 and Grade 2 | • Perfusion parameters: arterial flow, blood volume, permeability measured as ktrans |
• Clinical response was positively correlated to decrease in tumor permeability (P=0.03) after one and three cycles of nCxT. A cut-off of 25% has a sensitivity of 69% and a specificity of 58% |
| • Interim evaluation (median20 days) | • Histological response correlated with decrease in tumor permeability (P=0.03) and volume (P=0.03) only after 3 cycles of nCxT | |||||||
| • Post CxT (median 79 days; 6 days prior surgery) | • Non-statistical difference in arterial flow and blood volume | |||||||
| Blank |
Gastric and GEJ (Siewert I-II-III) cancer | 686 Cohort A 184 Cohort B | AC | Ct | • Baseline | Becker regression score ( |
Clinical response: combination of CT parameters (decrease of maximal transverse diameter <50%) and Endoscopic parameters (decrease of the endoluminal tumor size of >75%) | • Accuracy between clinical response and histopathological TRG =85% in Cohort A and 92% in Cohort B for NR and 52% in Cohort A and 50% in Cohort B for R |
| • Interim evaluation (118 pt) after 4–6 weeks of Ct | • Sensitivity, specificity, PPV and NPV for histopathological response respectively 60.5%, 80.2%, 51.9% and 85.2% in Cohort A and 66.7%, 85.4%, 50% and 92.1% in Cohort B | |||||||
| • Post NT | • Cohort A: clinical response statistically significant for prognosis in all localizations; histopathological regression only in AEG I-II; clinical response was an independent prognostic factor (non-response HR for death 1.4; 95% CI, 1.0–1.8, P=0.032) | |||||||
| • Cohort B: sensitivity of interim response |
||||||||
| Late response | ||||||||
| Jones |
EC | 50 | 38 SCC; 12 AC | CRT | • Baseline | America Joint Committee on Cancer Responders ( |
Responders = pCR | • Post CRT CT overstaged 36% and understaged 20% pathologic T classification |
| • Post CRT (mean 73 days between scans) | • The post CRT: CT T classification did not correlate with the pathologic T classification (P=0.09) | |||||||
| • CT had a sensitivity of 65% and a specificity of 33% in evaluating the pathological response (PPV 58% and NPV 41%) using ECOG solid tumor response criteria | ||||||||
| • No significant correlation between radiographic and pathological stage (P=0.83), tumor pCR status (P=0.22) or tumor histology (P=0.59) | ||||||||
| • No difference in tumor location or histology | ||||||||
| Konieczny |
EC and GEJ cancer | 35 (20 EC; 15 GEJ) | 25 AC; 10 SCC | CxT or CRT | • Baseline | Mandard score (tumor regression grade defined only in 25/35) | -Tumor depth |
• T stage correctly predicted in 12 of the 35 patients (34%) |
| • Post nCT (4–5 weeks) | • The tumor regression grade predicted correctly in 8% (2/25) patients; the degree of regression was overestimated in 24% and underestimated in 68% | |||||||
| • Complete CT response in predicting pCR: sensitivity 20%, specificity 96%, PPV 67%, NPV 75%, accuracy 74% | ||||||||
| Swisher |
EC and GEJ cancer (47 EC; 56 GEJ) | 103 | AC; SCC | CRT | • Baseline | Responders <10% viable cells | Esophageal wall thickness (67/103 patients with both CTs) | • Sensitivity, specificity and accuracy for pathological NR was respectively 51%, 69% and 62% on post CRT evaluation (threshold of oesophageal thickness ≥14.5 mm) |
| • 2–5 weeks after CRT | • Confirmed utility of PET, CT, and EUS to identify pathologic responders | |||||||
EC, esophageal cancer; GEJ, gastroesophageal junction; CxT, chemotherapy; CRT, chemoradiotherapy; EUS, ecographic ultrasound; CT, computed tomography; PET, positron emission tomography; R, responders; NR, non-responders; pCR, pathological complete responders; PPV, positive prognostic value; NPV, negative prognostic value; AC, adenocarcinoma; SCC, squamous cell carcinoma; HR, hazard ratio.
MRI
Differently from CT imaging, MRI provides a multiparametric, multiplanar assessment of the tumor burden with high soft tissue characterization.
Recent literature (9) suggests that the use of MRI is becoming increasingly frequent in diagnosis and follow-up of GEJ tumors, mainly due to technical improvements (i.e., breath hold, cardiac gating sequences) and to the addition of new quantitative parameters [i.e., diffusion weighted imaging (DWI) and its corresponding reconstructed apparent diffusion coefficient (ADC) map, dynamic contrast enhanced (DCE) MRI], to purely anatomic (T1, T2 weighted) sequences, having an intrinsic high soft tissue contrast resolution enough to differentiate pathological wall layers. GEJ MRI requires minimal patient preparation to properly depict the multilayer pattern of the gastroesophageal tract; intramuscular scopolamine (in the absence of contraindications) results beneficial as does proper visceral distension through the administration of at least 500 mL of water after a 6-hour fasting (9).
DWI, based on the random Brownian motion of water molecules within a voxel of tissue, is sensitive to microstructural changes which occur earlier than anatomical changes during nCRT. In a prospective cohort of 32 patients with biopsy proven GEJ locally advanced tumors, De Cobelli et al. (22) found that a post treatment ADC absolute value higher than 1.84×10−3 mm2/s and an increase of ADC percentage higher than 13.6% are useful findings in identifying pathological responders. The same authors demonstrated a strong inverse correlation between ΔADC (delta before and after nCRT) and tumor regression grade (TRG), regardless of any dimensional modification. A recent meta-analysis (23) including 236 patients substantially confirmed these observations (pooled sensitivity and specificity for ΔADC in predicting pathological response were 93% and 85%, respectively).
Similar imaging protocols have also shown to be able to differentiate responders from non-responders even after few cycles of nCRT. Weber et al. (24) used DWI-MRI to assess early response assessment of GEJ neoplasms, demonstrating that an increase in ADC absolute values after the first two weeks of nCRT was associated with 100% sensitivity and 50% specificity in identifying metabolic responders. A more recent meta-analysis (25) which includes 158 patients demonstrated that a relative increase of ADC values of approximately 21% after two to three weeks of neoadjuvant treatment correlates with favorable pathological response.
In a multicenter, international prospective study (26), patients scheduled to receive nCRT prior to resection were evaluated at three time points using DWI MRI scans (prior to, during and after nCRT). The authors found that relative changes in DWI parameters during nCRT were significantly different between responders and non-responders.
Giganti et al. (27) further explored the role of DWI in early prediction of responders and non-responders, using data from imaging prior to start of nCRT. The authors found that pathological responders have significantly lower pre-nCRT ADC absolute values than non-responders (1.32±0.331×10−3vs. 1.47±0.407×10−3 mm2/s). Possibly, the biological rationale of such a finding is that the higher the cellularity, the lower the ADC values but also the greater cytotoxic effect.
DCE MRI allows quantification of tumor perfusion and permeability. In a cohort of 26 patients with locally advanced esophageal and GEJ neoplasms, Heethuis et al. (28) found that DCE MRI changes, evaluated throughout treatment in the so-called tumor area under the concentration time curve (AUC), well correlated with pathological response. The same authors recently reported (29) that combining DWI and DCE MRI parameters, a more accurate assessment of tumor response to neoadjuvant treatment may be assessed.
Table 2 tabulates the selected studies assessing the role of MRI in neoadjuvant treatment response in GEJ cancer.
Table 2
| Study | Localization | No. patients | Histology | Neoadjuvant therapy | Timing of scans | Pathological assessment | MRI modality | MRI parameters | Results |
|---|---|---|---|---|---|---|---|---|---|
| Early and late response | |||||||||
| De Cobelli |
EC, gastric and GEJ cancer: (7 EC; 16 gastric; 9 GEJ) | 32 | 26 AC; 6 SCC | CxT or CRT | • Baseline | Mandard score ( |
1.5 T MRI with DWI cardiac and respiratory gated sequences (b value 0-600 s/mm2) | ADC (mean of ADC of each section of the tumor excluding necrotic area) | • No differences in tumor volume values between R and NR |
| • Post NT (median 10±3 days) | • Pre-NT ADC, post-NT ADC, ΔADC | • Significant differences were found evaluating ADC, with lower pre-NT values and significant increase after NT in R | |||||||
| • Tumor volume: post-NT V, ΔV | • Highly significant strong inverse correlation between ΔADC and TRG values (r=−0.71; P=0.000004); no evidence of correlation between ΔV and TRG (r=−0.02; P=0.883) | ||||||||
| • Pre-NT ADC cut off <1.5×10-3 mm2/s: R detected with a sensitivity of 35.29%, specificity of 60%, PPV of 50%, NPV of 50% and accuracy of 46.87% | |||||||||
| • ΔV cut-off of 57% decrease: R detected with a sensitivity of 35.29%, specificity of 66.66%, PPV of 55.54%, NPV of 47.16% and accuracy of 50% | |||||||||
| • Post-NT ADC cut-off of >1.84×103 mm2/s: R detected with a sensitivity of 70.6%, specificity of 80%, PPV of 80%, NPV of 70.6% and accuracy of 75% | |||||||||
| • ΔADC cut-off of 13.6% increase: R detected with a sensitivity of 88.2%, specificity of 86.7%, PPV of 88.2%, NPV of 86.7% and accuracy of 87.5% | |||||||||
| Cheng |
EC, gastric and GEJ cancer | 236 (7 studies) | AC; SCC | CxT or CRT | • Baseline | Pathological assessment in 4/7 studies | 1.5 T MRI (6/7 studies, one not specified): DWI with variable b value from 0 to 1,000 s/mm2 | ADC values measured from 3D data in 3/7 studies, from 2D data in 2/7 and not specified in 2/7 | • ΔADC: pooled sensitivity, specificity, DOR and AUC of 93% (95% CI, 77–98%), 85% (95% CI, 72–93%), 78 (95% CI, 15–401) and 0.91 (95% CI, 0.89–0.94) |
| • Post NT | • Post-ADC: pooled sensitivity, specificity, DOR and AUC of 75% (95% CI, 62–84%), 90% (95% CI, 67–97%), 26 (95% CI, 6–110) and 0.85 (95% CI, 0.82–0.88) | ||||||||
| Weber |
GEJ cancer (Siewert I-II) | 15 | AC | CxT (14 days), followed by CxT or CRT based on metabolic PET response (cut-off of SUV decrease of ≥35%) | MRI and FDG-PET/CT | Becker score (R: grade Ia-Ib-II) | 1.5 T MRI with respiratory gated DWI sequences (b value 50-400-800 s/mm2) | Mean of ADC values from 4 manual ROI (at least 80 pixels) avoiding necrotic areas | • Concordance of ADC increase and PET response observed in 73.3% of all patients |
| • Baseline | • The ADC at first MRI and the tumor SUV at first PET/CT were not different in PET- R (SUV decrease of ≥35%) and NR; increase in ADC was significantly higher in PET-R (26.8%±22.2%) than in PET NR (6.5%±15.8%, P=0.0298) | ||||||||
| • Post NT (after 14 days) | • ADC increase yielded a sensitivity, specificity, PPV and NPV respectively of 100%, 50%, 75% and 100% (cut-off) | ||||||||
| • GEJ with histological response had higher initial ADC values but not significant differences of ADC increase, initial SUV and SUV decrease | |||||||||
| • Non statistically significant differences in initial ADC and complete response (grade 1a) | |||||||||
| Maffazzioli |
EC | 158 (7 studies) | AC;SCC | CxT or CRT | • Baseline (6/7) | Mandard score in 5/7 studies (2/7 not specified): | • 4/7 studies 1,5 T MRI | ADC values measured from 3D data in 4/7 studies, from 2D data in 1/7 and not specified in 2/7 studies | • On pooled evaluation, baseline ADC was not significantly associated with pathological response (MD 0.11, 95% CI, 0.21–0.42; I2=85%; P<0.01) |
| • Interim evaluation (4/7) | • complete response (pCR, TRG1) | • 1/7 studies 3 T MRI | • PreNT ADC | • Two studies evaluated the differences in baseline ADC in pCR versus non pCR patients; in this subgroup analysis baseline ADC was significantly lower in pCR than non pCR patients | |||||
| • Post NT (7/7) | • good response (GR, TRG1-2) | • 2/7 studies not specified | • ΔADC from baseline to interim evaluation | • On pooled evaluation, a relatively increase in ADC at the interim evaluation of 21.06% was observed among responders (MD 21.06%; 95% CI, 13.04–29.09; I2=49%; P=0.12). A similar increase was identified in pCR versus non pCR subgroups | |||||
| • DWI (b value 0-200-800 in 3/7 studies; 0-600 and 0-700 in 2/7 studies, 2/7 not specified) | • ΔADC from baseline to postNT evaluation | • On pooled evaluation, a relatively increase in ADC at the post NT evaluation was observed among responders (MD 22.49% 95% CI, 9.98–35.05; I2=0%; P=0.46) | |||||||
| Borggreve |
EC | 69 | 57 AC; 11 SCC; 1 Undifferentiated large cells carcinoma | CRT | MRI and FDG-PET/CT: | Histological evaluation according to ( |
MRI with DWI (b value 0-200-800 s/mm2) | ADC: | • Patients with SC had a significantly higher probability of pCR and GR than AC patients |
| • Baseline | • mean ADC value | • The ΔADC between baseline and interim evaluation was associated with pCR (median, IQR: 28% [15%, 39%] for pCR versus 11% [4%, 17%] for non pCR, P=0.008), while ΔADC between baseline and post CRT evaluation were not statistically different. Same characteristics were found for R versus non-complete R | |||||||
| • Interim evaluation (median of 13 days after initiation of CRT) | • ΔADC between baseline and interim evaluation | • Complementary role of FDG-PET/TC and DWI MRI for pCR prediction: ROC analysis showed that ΔADC between baseline and interim evaluation combined with ΔSUV between baseline and interim evaluation and histology have a superior bootstrapped c-statistic in comparison with their individual value and histology (0.83; 95% CI, 0.74–0.94), with the lowest AIC | |||||||
| • Post NT | • ΔADC between baseline and post CRT evaluation | • No imaging parameters nor histology was associated to OS and DFS | |||||||
| Giganti |
EC and GEJ cancer (Siewert I) | 23; 9/23 CRT; 14/23 direct surgery | AC (9/23; 3/9CRT) SCC (14/23; 6/9 CRT) | CRT | • Baseline | Histological evaluation according to 7th TNM edition ( |
1.5 T MRI with DWI sequences (b value 0–600 s/mm2) | ADC (3D evaluation avoiding necrotic areas) | • Baseline ADC ≤1.4×10-3 mm2/s predicts negative prognosis in total population (P=0.016) and surgical group (P<0.001) |
| • Post CRT | |||||||||
| Heethuis |
EC and GEJ cancer (EC34; GEJ 11) | 45 | 38 AC; 5 SCC; 2 ASC | CRT | • Baseline | Mandard score | 1.5 T MRI: | ADC and area under the concentration time curve (AUC): | • DW-MRI P75 ΔADC between post-NT and pre-NT was most predictive for GR (c-index =0.75) |
| • Interim evaluation (weeks 2–3) | • pCR = TRG 1 | • free breathing DWI sequences (b value 0-200-800 s/mm2) | • Mean | • DCE-MRI P90 ΔAUC between interim and pre-NT was most predictive for pCR (c-index =0,79); relative increase in tumor AUC of 10.6%±17.6% for partial R versus 45.2%±41.5% for partialNR | |||||
| • Post NT (3–9 weeks after CRT) | • GR = TRG 1-2 | • DCE sequences | • Median | • Complementary value of DWI an DCE for pCR prediction (c-index =0.89) by multivariable logistic regression analysis | |||||
| • Several percentiles (P75/P90) | |||||||||
| • Δ between time points | |||||||||
CxT, chemotherapy; CRT, chemoradiotherapy; R, responders; NR, non-responders; ADC, apparent diffusion coefficient; DWI, diffusion weighted imaging; DCE, dynamic contrast enhanced; NT, neoadjuvant therapy; AC, adenocarcinoma; SCC, squamous cell carcinoma; ASC, adenosquamous carcinoma; V, volume; pCR, complete pathologic response.
PET
Fluorine18 fluorodeoxyglucose PET/CT (18F-FDG PET/CT) is a functional imaging modality that allows non-invasive characterization of physiologic and pathologic process. 18F-FDG PET/CT is very promising tool to assess in vivo metabolic response to therapy, as measured by tumor glucose metabolic treatment-induced changes. PET has been proposed as a quantitative measure of response to neoadjuvant therapy for patients with GEJ and esophageal carcinoma, both as end-of-treatment evaluation and as early assessment of response during treatment.
In the setting of evaluation at the end of treatment, Kauppi et al. (32) investigated the value of 18F-FDG PET/CT in predicting histopathological response, overall survival and disease survival. They evaluated 66 patients treated with nCxT for locally advanced carcinoma of the esophagus or GEJ. 18F-FDG PET/CT was performed before and after completion of neoadjuvant therapy, with standardized uptake value (SUV) being assessed for both scans to evaluate its relative change (SUVΔ%). Authors demonstrated that a change in baseline SUVΔ >67% was able to optimally predict histopathological response (sensitivity: 79% and specificity: 75%), being also associated with improved overall survival and disease-free survival.
Hernandez et al. (33) found a significant correlation between SUVmax response and histologic response in patients with locally advanced GEJ adenocarcinoma or gastric cancer. However, disease specific survival was only predicted by histopathologic response and tumour staging, but not by SUVmax.
Lately, Gabrielson et al. (34) found a significant reduction of standardized uptake ratio (SUR) in the primary tumour in histological responders compared to non-responders; furthermore, changes in SUR were significantly greater in responders following nCRT, but not following CxT alone.
Regarding the early assessment of response during treatment, Zum Büschenfelde (35) and his group carried on a prospective trial involving 56 patients with locally advanced adenocarcinomas of the GEJ who underwent 18F-FDG PET/CT before and 14 days after starting chemotherapy. The relevance of this trial relies on the possibility of using 18F-FDG PET as a tool for guiding treatment algorithm and provides changes in treatment strategy early in the course of chemotherapy.
Harustiak et al. obtained different results compared to Zum Büschenfelde (36). In order to assess the tumour early metabolic response to chemotherapy, 18F-FDG-PET/CT was performed before (PET1) and after (PET2) initiation of the first cycle of chemotherapy. Authors did not identify any association between median 𝚫SUL (SUV normalized to lean body mass) or median 𝚫TLG (total lesion glycolysis of the primary tumour) and histopathological response, thus concluding that 18F-FDG PET/CT does not predict histopathological response in patients with adenocarcinoma of the esophagus and GEJ after the first cycle of chemotherapy.
Similarly to the previous group, Schneider and colleagues (37) assessed the accuracy of 18F-FDG PET/CT in predicting the early pathologic response after neoadjuvant chemotherapy in 30 patients with locally-advanced gastric or GEJ cancer receiving nCxT. Metabolic response (defined as a decrease in SUV ≥35%) after nCxT was detected in 66.7% of patients, and among metabolic responders, 50% showed major and 50% minor pathologic regression. 18F-FDG PET/CT showed a sensitivity of 90.9%, specificity 47.3%, a positive predictive value 50%, a negative predictive value 90% and an accuracy of 63.3% as predictor of early response to neoadjuvant chemotherapy, having limited value in predicting overall pathologic response. However, the reliable detection of non-responders allowed the identification of those patients requiring an immediate change of therapy strategy (i.e., resection or modified multimodality therapy), similarly to zum Büschenfelde et al. (35).
Findlay et al. (38) investigated a different but interesting aspect regarding the possibility of using metabolic nodal stage (mN) and response (mNR) as new markers of disease progression, recurrence, and death in patients with esophageal or GEJ cancer undergoing neoadjuvant chemotherapy. The same group (39) performed a validation study on a cohort of patients with both esophageal and GEJ cancer, studied with 18F-FDG PET/CT before and after neoadjuvant chemotherapy. In patients undergoing successful resection, those without complete mNR presented worse prognosis (disease-free survival hazard ratio =2.46; P=0.004). Interestingly, these associations were independent of primary tumor metabolic, pathological response, and stage. The absence of complete mNR predicted recurrence or death at 1 and 2 years, with positive predictive values of 44.4% and 74.1%, respectively. This study suggests that mNR may provide surrogate information on phenotype of metastatic cancer clones beyond the mere presence of nodal metastases, and therefore its use might be suggested in order to better stratify patients and provide personalize treatments, including adjuvant therapy.
In Table 3 the selected studies assessing the role of PET in neoadjuvant treatment response in GEJ cancer are reported.
Table 3
| Study | N. Pts | Histology | Neoadjuvant therapy | Timing of scans | Pathological assessment | PET parameters assessed | Results |
|---|---|---|---|---|---|---|---|
| Early response | |||||||
| Zum Büschenfelde |
56 | AC | CxT | Baseline and 14 days after starting CxT | Histopathologic R (≤10% residual tumor) histopathologic NR (≥10% residual tumor) | Metabolic responders: mean SUV of 35% or more | 18F-FDG PET as possible tool for guiding treatment algorithm and providing changes in treatment strategy early in the course of chemotherapy |
| Harustiak |
126 | AC | CxT | Baseline and after a median of 16 days after the start of chemotherapy (range, 12–22) | As per Mandard criteria | Variation of peak SUL and TLG of the primary tumor between PET1 and PET2: ΔSUL and ΔTLG | • No association between median ΔSUL or median ΔTLG and histopathological response |
| • ΔTLG, but not ΔSUL, was associated with the histopathological response in a post hoc analysis of 47 pts with PET2 performed 16 days or less after the start of chemotherapy | |||||||
| ΔTLG was 66 per cent or more | |||||||
| Schneider |
30 (8 Gastric; 22 GEJ) | AC | CxT | Baseline and 14 days after starting CxT | Metabolic responders: decrease in SUV ≥35% | • 18F-FDG PET/CT reliably detect non-responders, thus allowing the identification of pts requiring an immediate change of therapy strategy (sens: 90.9%; spec: 47.3%; positive predictive value: 50%; negative predictive value: 90%; accuracy: 63.3%) | |
| Late response | |||||||
| Kauppi |
66 | AC | CxT | Baseline and at the end of treatment (median time from last T and PET: 15 days) | According to Schneider |
SUV Δ% [(SUV1- SUV2)/SUV1]x100 | • Change in baseline SUV >67% optimally predict histopathological response (sens 79%; spec: 75%) |
| • Change in baseline SUV >67% is associated with improved overall survival (HR 0.249, P=0.027) and disease-free survival (HR 0.383, P=0.040) | |||||||
| Hernandez |
192 (120 GEJ; 72 gastric) | AC | CxT or CXT and CRT | Baseline and at the end of treatment | – | SUVmax percentage change | • Significant correlation between SUVmax response and histologic response in patients with GEJ (rho =0.19, P=0.04) and gastric cancer (rho =0.44, P<0.0001) |
| • SUVmax response failed to demonstrate a relationship with DSS in multivariable models containing conventional pathologic variables | |||||||
| Gabrielson |
51 | AC | CxT or CXT and CRT | Baseline and at the end of treatment | TRG grading describing the ratio of tumor cells to fibrotic cells, as suggested by Chirieac |
SUR variation between baseline scan and post-treatment scan | • Significant SUR reduction in the primary tumor in histological responders compared to non-responders |
| Mean time between end of neoadjuvant therapy and follow-up PET/CT was similar in responders (15.7±9.2 days) and non-responders (17.9±24.9 days) | • Changes in SUR were significantly greater in responders following chemoradiotherapy, but not following chemotherapy alone | ||||||
| • No difference in SUR in patients with complete histological response compared to pts with subtotal response |
CxT, chemotherapy; CRT, chemoradiotherapy; AC, adenocarcinoma; GEJ, gastroesophageal junction; R, responders; NR, non responders; SUV, Standardized uptake volume; SUR, standardized uptake ratio (ratio between SUVmax of the tumor and SUVmean of a 1-cm3 VOI placed within the mediastinal blood pool); TLG, total lesion glycolysis; SUL, standardized uptake value normalized to lean body mass
The recent development of fully hybrid PET/MRI devices would represent the next step in hybrid imaging by combining the functional and metabolic characteristics of PET with the unique anatomical and functional information of MRI.
An interesting study by Belmouhand et al. (42) evaluated the feasibility of an early response assessment (3 weeks) to predict resectability using a hybrid 18F-FDG PET/MRI in patients treated with nCxT (n=22). Imaging identified 17 tumors as resectable and 5 as non resectable with a sensitivity and specificity of 94% and 80%, respectively for PET and MRI. Histopathology and RECIST were not correlated to resectability. This suggests that a multimodality imaging approach combining PET and MRI might provide complementary value for predicting pathologic response.
Radiomics
Radiomics is a novel tool consisting in extraction of quantitative data from medical images in order to develop predictive models relating imaging features to clinical outcomes. Only few studies incorporating radiomics in evaluation of treatment response in GEJ neoplasms exist, however early evidence suggests that imaging heterogeneity parameters could be prognostic.
In a cohort of 36 patients with contrast enhanced CT before and after nCRT, Yip et al. (43) reported that post treatment texture parameters are associated with OS; specifically, post treatment medium entropy of less than 7.356, coarse entropy of less than 7.116 and median uniformity of 0.007 or greater were associated with improved median OS, 33.2 vs. 11.7 months (P=0.0002). Furthermore, CT tumor heterogeneity decreases following nCRT in those patients with good response. The study also found that survival models which evaluated baseline (pre-treatment) texture parameters (entropy, uniformity) and maximal wall thickness perform better than maximal wall thickness alone in assessing survival.
Hou et al. (44) also found that CT based radiomic features can be used as imaging biomarkers to predict response to nCRT in an Asian population cohort with esophageal carcinoma.
Giganti et al. (45) studied pre-treatment first order energy, entropy, and skewness and found that they were significantly associated with a tumor aggressiveness and negative prognosis in 56 patients, supporting the claim that tumors with greater heterogeneity (e.g., higher entropy) are related to a worse outcome.
Table 4 tabulates the studies in which radiomic analysis was used to assess neoadjuvant treatment response in GEJ cancer.
Table 4
| Study | Localization | N. patients | Histology | Neoadjuvant therapy | Timing of scans | Response assessment | CT parameters | Results |
|---|---|---|---|---|---|---|---|---|
| Yip |
Esophageal cancer | 36 | 26 SCC; 9 AC | Definitive CRT | • Baseline | RECIST criteria ( |
Wall thickness; texture analysis: | • Post CRT entropy <7,356, coarse entropy <7,116 and median uniformity ≥0.007 were associated with improved OS (P<0.01) |
| • Post NT (median 65 days) | • entropy | • None of the baseline or changes in texture parameters after CRT nor morphological response assessment was associated with OS | ||||||
| • uniformity | • Survival models that combine pre-treatment entropy and uniformity with maximal wall thickness assessment, respectively, performed better than morphological assessment alone [AUC of 0.767 |
|||||||
| • mean grey level intensity | ||||||||
| • kurtosis | ||||||||
| • SD of the histogram | ||||||||
| • skewness | ||||||||
| Giganti |
Gastric and GEJ (Siewert II-III) cancer: | 56 | 37 AC; 19 Signet-ring cell | None | • Baseline | Texture parameters and OS | 107 radiomic features: | • Kaplan-Meier curves were significantly different for 58/107 features and, after adjustment, for 50/107 texture parameters |
| • 2 Siewert II | • fist-order texture analysis | • Energy, entropy [no filter], entropy [filter 1,5], maximum HU value and skewness were associated to a negative prognosis in a multivariate model, according to different thresholds | ||||||
| • 7 Siewert III | • second-order texture analysis | • Specifically, energy (2a), entropy [filter 1.5], maximum HU value, skewness, mean absolute deviation and root mean square were also predictors of OS at univariate analysis | ||||||
| • 47 Stomach | • shape and size features |
CRT, chemoradiotherapy; AC, adenocarcinoma; HU, Hounsfield Unit; OS, overall survival; AUC, area under curve.
Discussion
Current state of the art response assessment following neoadjuvant therapy in GEJ adenocarcinoma is suboptimal.
The few published studies specifically exploring the role of CT in assessing tumour response after nCRT in patients with GEJ adenocarcinoma have been inconclusive: changes in CT tumour dimension or volume do not represent a sensitive imaging biomarker in response evaluation. Furthermore, due to poor contrast resolution issue, CT is unable to adequately help differentiate between T1, T2 and T3 disease, compromising a precise downstaging assessment with low sensitivity and specificity (33–55% and 50–71%, respectively) (14).
Solutions to such issues could come from perfusion techniques: following neoadjuvant chemotherapy, normalization of tumor chaotic vasculature is hypothesized to occur, and it may reduce the pathological leakiness of the vessels and therefore decrease the extravasation of contrast agent from the intravascular compartment into the extracellular space. However, radiation could induce the release of proangiogenic factors and stimulate angiogenesis, thus impairing an optimal perfusion evaluation (14,15).
18F-FDG PET/CT has shown the most potential in this setting, since it can reliably discriminate early on between responder and non-responder status, thus providing information to choose the proper treatment strategy (35,37). Additionally, 18F-FDG PET/CT has been proven to adequately identify those patients with GEJ adenocarcinoma with worse prognosis after neoadjuvant chemotherapy completion (35). On the other hand, literature does indicate a limited value of 18F-FDG PET/CT in predicting overall pathological response (33). Furthermore, its accuracy could be affected by post treatment inflammation.
Potentially, a single imaging modality able to provide optimal soft tissue delineation together with functional information regarding tumour cellular proliferation, angiogenesis and microenvironment biology may be the best predictive and prognostic imaging tool. Early evidence suggests that MR, which provides a multiparametric, multiplanar assessment of the tumour burden with optimal soft tissue characterization (9) in association with an accurate depiction of functional modifications occurring early during neoadjuvant treatment, could play a major role in clinical practice. Specifically, recent literature highlights the role of DWI and DCE MRI, both providing intriguing insights into the biological environment of the tumour and on changes which occur therein during treatment.
Of note, the combination of PET and MRI, in particular when available as a full hybrid modality, could represent an optimal tool for evaluating GEJ treatment response after neoadjuvant treatment, since it might be able to both provide anatomical depiction as well as to quantify functional and metabolic information.
In this setting, imaging heterogeneity analysis will certainly represent an essential part of the overall treatment response assessment, even though further studies are needed.
In conclusion, imaging biomarkers can yield important information on tumour characterization and treatment response. However, overall prognosis of responders remains poor, suggesting underlying differences in tumour biology. Currently, data supports imaging biomarkers in detecting non-responders, which should be directly addressed to surgery without continuing neoadjuvant treatment.
A multimodal algorithm based on CT tissue density measures (dimensional evaluation), multiparametric MRI which can yield quantitative data, in particular ADC, 18-FDG PET/CT and FDG PET/MRI using SUV and metabolic tumor volume with information on aggressiveness derived from radiomics, could aid in correctly evaluating and potential standardizing through a validation evaluation of treatment response.
Acknowledgments
Funding: The research leading to these results has received funding from AIRC (Italian Association for Cancer Research) under Investigator Grant - IG 2019 - ID. 23015 project – P.I. De Cobelli Francesco; NCT04359732.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Riccardo Rosati) for the series “Current issues on GEJ adenocarcinoma” published in Annals of Esophagus. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at: http://dx.doi.org/10.21037/aoe-2020-geja-04). The series “Current issues on GEJ adenocarcinoma” was commissioned by the editorial office without any funding or sponsorship. MP reports other from GE Healthcare, outside the submitted work. FDC reports grants from AIRC (Italian Association for Cancer Research) during the conduct of the study. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rüdiger Siewert J, Feith M, Werner M, et al. Adenocarcinoma of the esophagogastric junction: results of surgical therapy based on anatomical/topographic classification in 1,002 consecutive patients. Ann Surg 2000;232:353-61. [Crossref] [PubMed]
- Knight WRC, Zylstra J, Van Hemelrijck M, et al. Patterns of recurrence in oesophageal cancer following oesophagectomy in the era of neoadjuvant chemotherapy. BJS Open 2018;1:182-90. [Crossref] [PubMed]
- van Hagen P, Hulshof MCCM, van Lanschot JJB, et al. Preoperative Chemoradiotherapy for Esophageal or Junctional Cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Shapiro J, Van Lanscho JJB, Hulshof MCCM, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. [Crossref] [PubMed]
- Stahl M, Walz MK, Riera-Knorrenschild J, et al. Preoperative chemotherapy versus chemoradiotherapy in locally advanced adenocarcinomas of the oesophagogastric junction (POET): Long-term results of a controlled randomised trial. Eur J Cancer 2017;81:183-90. [Crossref] [PubMed]
- Noordman BJ, Spaander MCW, Valkema R, et al. Detection of residual disease after neoadjuvant chemoradiotherapy for oesophageal cancer (preSANO): a prospective multicentre, diagnostic cohort study. Lancet Oncol 2018;19:965-74. [Crossref] [PubMed]
- Bain GH, Petty RD. Predicting response to treatment in gastroesophageal junction adenocarcinomas: combining clinical, imaging, and molecular biomarkers. Oncologist 2010;15:270-84. [Crossref] [PubMed]
- Borggreve AS, Mook S, Verheij M, et al. Preoperative image-guided identification of response to neoadjuvant chemoradiotherapy in esophageal cancer (PRIDE): a multicenter observational study. BMC Cancer 2018;18:1006. [Crossref] [PubMed]
- De Cobelli F, Palumbo D, Albarello L, et al. Esophagus and Stomach: Is There a Role for MR Imaging? Magn Reson Imaging Clin N Am 2020;28:1-15. [Crossref] [PubMed]
- Beer AJ, Wieder HA, Lordick F, et al. Adenocarcinomas of esophagogastric junction: multi-detector row CT to evaluate early response to neoadjuvant chemotherapy. Radiology 2006;239:472-80. [Crossref] [PubMed]
- Jones DR, Parker LA, Detterbeck FC, et al. Inadequacy of computed tomography in assessing patients with esophageal carcinoma after induction chemoradiotherapy. Cancer 1999;85:1026-32. [Crossref] [PubMed]
- Konieczny A, Meyer P, Schnider A, et al. Accuracy of multidetector-row CT for restaging after neoadjuvant treatment in patients with oesophageal cancer. Eur Radiol 2013;23:2492-502. [Crossref] [PubMed]
- van Heijl M, Phoa SS, van Berge Henegouwen MI, et al. Accuracy and reproducibility of 3D-CT measurements for early response assessment of chemoradiotherapy in patients with oesophageal cancer. Eur J Surg Oncol 2011;37:1064-71. [Crossref] [PubMed]
- Yip C, Cook GJ, Landau DB, et al. Performance of different imaging modalities in assessment of response to neoadjuvant therapy in primary esophageal cancer. Dis Esophagus 2016;29:116-30. [Crossref] [PubMed]
- Giganti F, Ambrosi A, Petrone MC, et al. Prospective comparison of MR with diffusion-weighted imaging, endoscopic ultrasound, MDCT and positron emission tomography-CT in the pre-operative staging of oesophageal cancer: results from a pilot study. Br J Radiol 2016;89:20160087 [Crossref] [PubMed]
- Lundsgaard Hansen M, Fallentin E, Lauridsen C, et al. Computed tomography (CT) perfusion as an early predictive marker for treatment response to neoadjuvant chemotherapy in gastroesophageal junction cancer and gastric cancer--a prospective study. Plos one 2014;9:e97605 [Crossref] [PubMed]
- Blank S, Lordick F, Bader F, et al. Post-therapeutic response evaluation by a combination of endoscopy and CT scan in esophagogastric adenocarcinoma after chemotherapy: better than its reputation. Gastric Cancer 2015;18:314-25. [Crossref] [PubMed]
- Becker K, Mueller JD, Schulmacher C, et al. Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer 2003;98:1521-30. [Crossref] [PubMed]
- Mandard AM, Dalibard F, Mandard JC, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer 1994;73:2680-6. [Crossref] [PubMed]
- American Joint Committee on Cancer. AJCC cancer staging handbook, 3rd Edition. J.B. Lippincott Company, Philadelphia, 1988.
- Swisher SG, Maish M, Erasmus JJ, et al. Utility of PET, CT, and EUS to Identify Pathologic Responders in Esophageal Cancer. Ann Thorac Surg 2004;78:1152-60. [Crossref] [PubMed]
- De Cobelli F, Giganti F, Orsenigo E, et al. Apparent diffusion coefficient modifications in assessing gastro-oesophageal cancer response to neoadjuvant treatment: comparison with tumor regression grade at histology. Eur Radiol 2013;23:2165-74. [Crossref] [PubMed]
- Cheng B, Yu J. Predictive value of diffusion-weighted MR imaging in early response to chemoradiotherapy of esophageal cancer: a meta-analysis. Dis Esophagus 2019;32:doy065 [Crossref] [PubMed]
- Weber MA, Bender K, von Gall CC, et al. Assessment of diffusion-weighted MRI and 18F-fluoro-deoxyglucose PET/CT in monitoring early response to neoadjuvant chemotherapy in adenocarcinoma of the esophagogastric junction. J Gastrointestin Liver Dis 2013;22:45-52. [PubMed]
- Maffazzioli L, Zilio MB, Klamt AL, et al. ADC as a predictor of pathologic response to neoadjuvant therapy in esophageal cancer: a systematic review and meta-analysis. Eur Radiol 2020;30:3934-42. [Crossref] [PubMed]
- Borggreve AS, Goense L, van Rossum PSN, et al. Preoperative Prediction of Pathologic Response to Neoadjuvant Chemoradiotherapy in Patients with Esophageal Cancer Using 18F-FDG PET/CT and DW-MRI: A Prospective Multicenter Study. Int J Radiat Oncol Biol Phys 2020;106:998-1009. [Crossref] [PubMed]
- Giganti F, Salerno A, Ambrosi A, et al. Prognostic utility of diffusion-weighted MRI in oesophageal cancer: is apparent diffusion coefficient a potential marker of tumor aggressiveness? Radiol Med 2016;121:173-80. [Crossref] [PubMed]
- Heethuis SE, van Rossum PS, Lips IM, et al. Dynamic contrast-enhanced MRI for treatment response assessment in patients with oesophageal cancer receiving neoadjuvant chemoradiotherapy. Radiother Oncol 2016;120:128-35. [Crossref] [PubMed]
- Heethuis SE, Goense L, van Rossum PSN, et al. DW-MRI and DCE-MRI are of complementary value in predicting pathologic response to neoadjuvant chemoradiotherapy for esophageal cancer. Acta Oncol 2018;57:1201-8. [Crossref] [PubMed]
- Rice TW, Blackstone EH, Rusch VW. 7th Edition of the AJCC Cancer Staging Manual: Esophagus and Esophagogastric Junction. Ann Surg Oncol 2010;17:1721-4.
- Sobin LH, Gospodarowicz MK, Wittekind C. TMN Classification of Malignant Tumors, 7th edition. Wiley-Blackwell Hoboken, 2010.
- Kauppi JT, Oksala N, Salo JA, et al. Locally advanced esophageal adenocarcinoma: response to neoadjuvant chemotherapy and survival predicted by ([18F])FDG-PET/CT. Acta Oncol 2012;51:636-44. [Crossref] [PubMed]
- Hernandez JM, Beylergil V, Goldman DA, et al. Post-Treatment/Pre-operative PET Response Is Not an Independent Predictor of Outcomes for Patients With Gastric and GEJ Adenocarcinoma. Ann Surg 2018;267:898-904. [Crossref] [PubMed]
- Gabrielson S, Sanchez-Crespo A, Klevebro F, et al. 18F FDG-PET/CT evaluation of histological response after neoadjuvant treatment in patients with cancer of the esophagus or gastroesophageal junction. Acta Radiol 2019;60:578-85. [Crossref] [PubMed]
- zum Büschenfelde CM, Herrmann K, Schuster T, et al. 18F-FDG PET–Guided Salvage Neoadjuvant Radiochemotherapy of Adenocarcinoma of the Esophagogastric Junction: The MUNICON II Trial. J Nucl Med 2011;52:1189-96. [Crossref] [PubMed]
- Harustiak T, Zemanova M, Fencl P, et al. [18F]Fluorodeoxyglucose PET/CT and prediction of histopathological response to neoadjuvant chemotherapy for adenocarcinoma of the oesophagus and oesophagogastric junction. Br J Surg 2018;105:419-28. [Crossref] [PubMed]
- Schneider PM, Eshmuminov D, Rordorf T, et al. 18FDG-PET-CT identifies histopathological non-responders after neoadjuvant chemotherapy in locally advanced gastric and cardia cancer: cohort study. BMC Cancer 2018;18:548. [Crossref] [PubMed]
- Findlay JM, Gillies RS, Franklin JM, et al. Restaging oesophageal cancer after neoadjuvant therapy with (18)F-FDG PET-CT: identifying interval metastases and predicting incurable disease at surgery. Eur Radiol 2016;26:3519-33. [Crossref] [PubMed]
- Findlay JM, Dickson E, Fiorani C, et al. Temporal validation of metabolic nodal response of esophageal cancer to neoadjuvant chemotherapy as an independent predictor of unresectable disease, survival, and recurrence. Eur Radiol 2019;29:6717-27. [Crossref] [PubMed]
- Schneider PM, Baldus SE, Metzger R, et al. Histomorphologic tumor regression and lymph node metastases determine prognosis following neoadjuvant radiochemotherapy for esophageal cancer: Implications for response classification. Ann Surg 2005;242:684-92. [Crossref] [PubMed]
- Chirieac LR, Swisher SG, Ajani JA, et al. Post therapy pathologic stage predicts survival in patients with esophageal carcinoma receiving preoperative chemoradiation. Cancer 2005;103:1347-55. [Crossref] [PubMed]
- Belmouhand M, Löfgren J, Johannesen HH, et al. Early response evaluation of neoadjuvant therapy with PET/MRI to predict resectability in patients with adenocarcinoma of the esophagogastric junction. Abdom Radiol (NY) 2019;44:836-44. [Crossref] [PubMed]
- Yip C, Landau D, Kozarski R, et al. Primary Esophageal Cancer: Heterogeneity as Potential Prognostic Biomarker in Patients Treated with Definitive Chemotherapy and Radiation Therapy. Radiology 2014;270:141-8. [Crossref] [PubMed]
- Hou Z, Ren W, Li S, et al. Radiomic analysis in contrast-enhanced CT: predict treatment response to chemoradiotherapy in esophageal carcinoma. Oncotarget 2017;8:104444-54. [Crossref] [PubMed]
- Giganti F, Antunes S, Salerno A, et al. Gastric cancer: texture analysis from multidetector computed tomography as a potential preoperative prognostic biomarker. Eur Radiol 2017;27:1831-9. [Crossref] [PubMed]
- Schwartz LH, Litière S, de Vries SE, et al. RECIST 1.1 – Update and Clarification: From the RECIST Committee. Eur J Cancer 2016;62:132-7. [Crossref] [PubMed]
Cite this article as: Palumbo D, Mapelli P, Nicoletti V, Steidler S, Picchio M, De Cobelli F. Imaging in evaluation of response to neo-adjuvant treatment. Ann Esophagus 2020;3:38.