Pneumatic balloon dilatation for achalasia—how and why I do it
Introduction
Hydrostatic dilatation for “cardiospasm” was first described by Plummer in 1908, with successful outcomes reported in a subsequent series by the same author in 1912 (1). Despite the antiquity of the treatment, there are several clinical scenarios where pneumatic balloon dilatation remains useful. In the patient who has previously had an operative cardiomyotomy, balloon dilatation is simpler than re-operative surgery and delivers good results. Similarly, in a patient who has had previous surgery in the left upper quadrant of the abdomen, particularly open surgery, balloon dilatation avoids the potential challenges of extensive adhesions preventing access to the hiatus. Likewise, in the morbidly obese with fatty liver and an enlarged left lobe, an endoscopic balloon approach remains straight forward. While the per-oral endoscopic myotomy (POEM) procedure is an alternative endoscopic treatment in such situations, it requires advanced endoscopic skills and has a significant learning curve which is not easily attained with such a low incidence condition as achalasia. Hence, pneumatic dilatation continues to have a role in the management of achalasia patients.
How I do it
Balloon dilatation for achalasia can be safely undertaken as an outpatient procedure in most patients. It requires a patient properly consented to the procedural risks of bleeding, perforation, and aspiration events. It also requires that the patient understands there is a significant chance that the procedure may need to be repeated in future should the initial therapeutic result fail to be satisfactory and/or durable.
The patient must be fasted, and in the setting of achalasia this often requires a more extended period without solid food to avoid performing a procedure on a patient with a very large volume of esophageal food debris, which is tedious to clear and increases the risk of peri-procedural aspiration. The author’s preference is for the patient to cease all solid food 48 hours prior to the procedure and take oral fluids only up to the standard 6 hour fasting period.
While routine endoscopy is commonly performed with the proceduralist delivering the sedation, interventional endoscopy in the achalasia patient group requires anesthesia delivered by an anaesthetist. This relates both to the depth of sedation required to deliver balloon dilation without significant patient discomfort, and managing the risk of aspiration. Balloon dilation can be performed under either sedation (with the patient in the left lateral position) or general anesthesia (with the patient supine). This is really an anesthetic decision, and particularly relates to the concerns regarding aspiration, but fluoroscopy is certainly more easily performed with a patient in the supine position.
Once the patient is anesthetised, standard endoscopy is performed. Thorough lavage of any esophageal salivary or food residue should be performed to aid in assessment of the esophagus and decrease the risk of substantial mediastinal contamination should a perforation occur. The gastro-esophageal junction should be thoroughly inspected to assess the degree of spontaneous relaxation (if any) and rule out any other potential cause for esophago-gastric outflow obstructions (i.e., pseudo-achalasia).
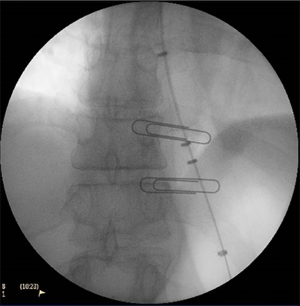
A Savary Gilliard wire is placed to the antrum and, as the scope is withdrawn under fluoroscopic guidance, radio-opaque markers can be placed just below and then above the gastro-esophageal junction. This enables confirmation that the dilating balloon is appropriately centred at the gastro-esophageal junction. The endoscope is withdrawn fully.
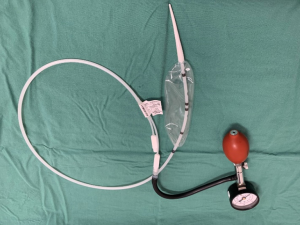
A Rigiflex™ Balloon (Boston Scientific, MA) is then inserted (Figure 1) over the guidewire and passed under fluoroscopic guidance until centred over the gastro-esophageal junction (Figure 2). The previously placed radio-opaque markers can be used in combination with the radio-opaque markers in the balloon itself to ensure accurate positioning. For all initial balloon dilatation procedures the balloon should be 30mm diameter, given the increased perforation risk associated with using the 35 or the 40mm balloon at the primary procedure (2).
The balloon is fully inflated via a hand held manometer and, on occasion, a waist in the middle of the balloon at the level of the gastro-esophageal junction can be appreciated, and observed to efface as the balloon effectively dilates the lower esophageal sphincter. The balloon often has a tendency to slip distally into the stomach and the balloon catheter needs to be held firmly by the operator to prevent this occurrence.
The Rigiflex™ Balloon is inflated for 60 seconds, then deflated; and the process repeated 3 times in total, with fluoroscopic guidance to confirm correct positioning. The balloon is then withdrawn, although it is advisable to leave the guidewire in situ. A check endoscopy is then performed in order to assess the degree of trauma at the gastro-esophageal junction and, in the setting of achalasia, there should be some mucosal trauma at least if the dilatation has been therapeutic. It is critical to rule out a full thickness perforation so that appropriate management can be instituted without delay. It is in the setting of a perforation that leaving the guidewire in situ can be invaluable, as it provides definite access to the stomach for a nasogastric drainage tube or an enteral feeding tube, which may not be so easy to navigate if there is significant bleeding or a perforation. If there is any doubt regarding the presence of a perforation, a water soluble contrast study should be performed (3).
Provided there has been no procedural complication, the scope and guidewire can both safely be withdrawn. The patient can be commenced on oral intake when sufficiently awake, and discharged within a few hours. The patient should be placed on a pureed diet for 48 hours and can then be moved onto a more normal diet.
The patient should be reviewed 2 to 3 weeks post-dilatation to assess the symptomatic outcome. If there are persistent symptoms of dysphagia, then the procedure should be repeated step-wise with a 35mm balloon, and if still not subsequently resolved, a 40 mm balloon. It is safe to perform repeat dilatation at 3–4 week intervals. In the situation of an achalasia patient with late recurrent symptoms post-balloon dilatation, it is reasonable to repeat the balloon dilatation with the same sized balloon used previously.
Results of balloon dilatation
The results of pneumatic balloon dilatation can be analysed in two ways: success based on the diameter of the balloon used, or success in comparison to other treatment modalities. A metanalysis (2) of the treatment success based on balloon size suggests that both 30 and 35 mm dilatation result in similar clinical improvement. However, the use of a 35 mm balloon for the initial dilatation has a higher rate of esophageal perforation than a 30mm balloon (9% vs. 1%). As well, patients who underwent graduated dilatations up to 40mm achieved better symptom resolution than patients taken only to 30 or 35 mm.
Pneumatic balloon dilatation has been compared to other interventional achalasia treatment options. Boeckxstaens et al. (4) randomised 201 patients to either laparoscopic cardiomyotomy or pneumatic balloon dilatation. They reported no significant difference between the success of either treatment at 1 or 2 year follow-up when measured by Eckardt score. Those treated with pneumatic balloon dilatation required more repeat interventions to maintain success, generally with stepping up the balloon size. In the initial phase of the study when a 35mm balloon was used at the first dilatation, a high rate of perforation occurred (4 of 13 patients), resulting in a change of protocol where the 30mm balloon was always used for the first dilatation. It was noted that increased age was associated with an increased perforation rate.
At subsequent long-term (5 year) follow-up of patients from the same trial patient group, again no significant difference between pneumatic dilatation and surgical myotomy was observed (5). In a metanalysis of reported outcomes based on achalasia subtypes, pneumatic balloon dilation was found to be equivalent to laparoscopic cardiomyotomy in type II achalasia, but inferior in types I and III (6).
In a recent randomised trial (7) of pneumatic dilatation compared to POEM the success rate as measured by Eckardt score favoured POEM over pneumatic dilatation when measured at 2 years (92% vs. 54%). With the pneumatic dilation patients often receiving multiple dilatations to achieve symptomatic improvement, that group had more than double the number of treatments compared to the POEM group. Perhaps as expected, with a more effective disruption of the lower esophageal sphincter, a higher rate of reflux esophagitis was found in the POEM patients than in the patients treated with pneumatic dilatation (46% vs. 11%). With no studies on POEM reporting long-term follow-up, the clinical significance of such a high rate of esophagitis is not clear, but is of concern.
For patients who have previously undergone a laparoscopic Hellers myotomy presenting with recurrent symptoms, pneumatic dilatation is a sound treatment option. While the evidence is largely limited to case series, the results of pneumatic dilatation in this setting seem equivalent to those in patients with primary achalasia, and similar to either a redo laparoscopic cardiomyotomy or POEM, without some of the technical challenges the latter 2 procedures present (8).
Conclusions
Pneumatic balloon dilatation for achalasia can be performed in an outpatient setting with acceptable results. It generally requires more than one treatment session, and both clinicians and patients need to be aware of this when making a decision about treatment type. While laparoscopic cardiomyotomy remains the first line surgical treatment, pneumatic dilatation remains a useful therapeutic option for clinicians treating patients with achalasia, particularly in situations where a cardiomyotomy has already been performed, or where surgical access may be difficult.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Sarah Thompson) for the series “Achalasia” published in Annals of Esophagus. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoe.2020.04.02). The series “Achalasia” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Plummer HS. Diffuse dilatation of the esophagus without anastomotic stenosis (cardiospasm). A report of 91 cases. JAMA 1912;58:2013-15. [Crossref]
- van Hoeij FB, Prins LI, Smout AJPM, et al. Efficacy and safety of pneumatic dilation in achalasia: A systematic review and meta-analysis. Neurogastroenterol Motil 2019;31:e13548 [Crossref] [PubMed]
- Zaninotto G, Bennett C, Boeckxstaens G, et al. The 2018 ISDE achalasia guidelines. Dis Esophagus 2018; [Crossref] [PubMed]
- Boeckxstaens GE, Annese V, des Varannes SB, et al. Pneumatic dilation versus laparoscopic Heller's myotomy for idiopathic achalasia. N Engl J Med 2011;364:1807-16. [Crossref] [PubMed]
- Moonen A, Annese V, Belmans A, et al. Long-term results of the European achalasia trial: a multicentre randomised controlled trial comparing pneumatic dilation versus laparoscopic Heller myotomy. Gut 2016;65:732-9. [Crossref] [PubMed]
- Andolfi C, Fisichella PM. Meta-analysis of clinical outcome after treatment for achalasia based on manometric subtypes. Br J Surg 2019;106:332-41. [Crossref] [PubMed]
- Ponds FA, Fockens P, Lei A, et al. Effect of Peroral Endoscopic Myotomy vs Pneumatic Dilation on Symptom Severity and Treatment Outcomes Among Treatment-Naive Patients With Achalasia: A Randomized Clinical Trial. JAMA 2019;322:134-44. [Crossref] [PubMed]
- Fernandez-Ananin S, Fernández AF, et al. What to do when Heller's myotomy fails? Pneumatic dilatation, laparoscopic remyotomy or peroral endoscopic myotomy: A systematic review. J Minim Access Surg 2018;14:177-84. [Crossref] [PubMed]
Cite this article as: Bright T. Pneumatic balloon dilatation for achalasia—how and why I do it. Ann Esophagus 2020;3:25.