
Free jejunal flap esophagoplasty for ischemic colon conduit replacement
Esophageal reconstruction using colon segments has two main indications: (I) in case of benign diseases (corrosive stricture, achalasia) the preservation of the stomach results in better digestion, therefore a colon substitute is the best option; (II) in case of esophageal cancer, the first choice is the stomach, however if the stomach has been resected or is found to be unsuitable due to other reasons, the colon has to be used. The colon is capable of bridging a long section and has a reliable blood supply. However, early and late ischemic complications can occur between 5–15% (1-3). There are three major forms of ischemic complications: (I) anastomosis insufficiency followed by stenosis (II) necrosis of the colon conduit (III) late ischemic stricture of the colon conduit. Anastomosis complications can be treated conservatively or surgically. Conduit necrosis is a devastating complication and in severe cases acute resection is required, however reconstruction can be delayed depending on the patient’s condition. Colonic stricture due to chronic ischemia can be treated with conservative methods such as dilation and stenting. In case of unsuccessful conservative methods surgery may be indicated since the impaired deglutition compromises the quality of life. If the stomach is unsuitable for replacement after a colon conduit necrosis, the best option is a jejunal flap transplantation. The jejunum has strong peristalsis with no significant secretion, the diameter of lumen is similar to the esophagus and its malignant transformation is uncommon. Jejunum can rarely be used for the reconstruction of extensive esophageal defects in European population due to the short mesentery, however the deficiency of vascular supply can be overcome by a free flap technique or by a microvascularly augmented supercharged Roux-en-Y limb. Microvascular anastomosis of the free flap is safely made by highly skilled plastic surgeons. We present three cases of complex jejunal reconstructions after the failure of a previous ischemic colon conduit, with different approaches in each case.
Case presentation
Substernal route—patient 1
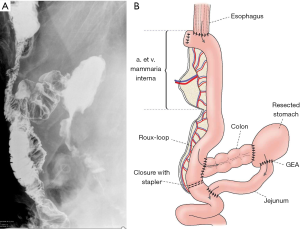
A 64-year-old male, diagnosed with T3N1M0 stage squamous cell carcinoma of the esophagus, was treated with neoadjuvant therapy resulting in complete remission. He had a Billroth II gastric resection in his history twenty years earlier. Two months after the successful oncological therapy, we performed transhiatal esophagectomy and reconstructed the defect using a left colon conduit pulled up through the posterior mediastinum. On the tenth postoperative day, he became septic and salivatory fistula appeared through the neck wound. After opening the wound, the colon conduit appeared to be necrotic. The cervical anastomosis of the conduit was taken down with a proximal esophageal diversion. After retracting the transplanted colon into the abdomen, the proximal portion was confirmed to be necrotic. This portion was resected, its end was closed blindly, and the remnant of the conduit was transferred subcutaneously to its highest possible presternal position. A catheter jejunostomy was created. The patient recovered. The reconstruction was made one year later. The mesentery of the jejunum was surprisingly mobile with strong arcades, so three straight arterial branches could be ligated, still it could not reach up to the neck. To obtain the required length of the graft, the vascular arch was interrupted between the second and third branch to create a “hybrid” supercharged Roux limb. This way the required length could be achieved, to bridge the gap. A 1 cm portion of the cartilage of right 4th rib was resected through a parasternal incision creating a small window to identify the internal mammary artery and vein. The jejunum was substernally pulled up to the neck with its original vasculature preserved distally (1st and 2nd branch). The interrupted vascular pedicle (3rd branch) could then be pulled out through the parasternal window for the planned microvascular anastomosis. The arterial branch was anastomosed end-to-end type to the internal mammary artery using 8-0 non-absorbable sutures. The internal mammary vein had a small caliber, so a 15 cm long saphenous vein graft was isolated from the leg to interconnect the mesenterial vein to the right external jugular vein through a subcutaneous tunnel. Then, the esophagojejunostomy was created in an end-to-end fashion with 3-0 absorbable sutures. The jejunocolostomy was performed in a side-to-end fashion and the Roux limb was transected with a stapler right below this anastomosis. This way a detour was done which redirected the passage into the resected stomach through the remaining colon conduit. The jejuno-jejunostomy was performed right below the transected segment of the jejunum. The patient was discharged on the twelfth day after an uneventful postoperative period. The contrast swallow (Figure 1) proved the patency of the conduit, passing freely into the Roux limb directed to colon, then to the resected stomach. The patient has been living for five years with no complications ever since.
Presternal route—patient 2
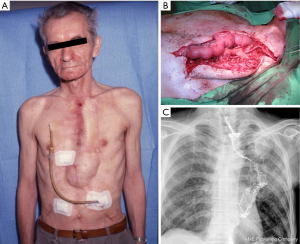
A 53-year-old male had an Ivor-Lewis surgery elsewhere because of a middle third esophageal cancer. Postoperatively, the stomach used for the reconstruction had partially necrotized, thus it was subsequently resected from an abdominal approach and a cervical esophagostomy and a gastrostomy was performed. The patient survived and after six months was admitted to our department. Reconstruction was performed using a right ileocolon pulled up substernally. On the fifth postoperative day, necrosis of the ileum was suspected. At the reoperation the cervical anastomosis was disconnected and an esophagostomy was placed. The colon conduit was then retracted to the abdomen, the proximal necrotic part was resected and the remnant of the colon conduit was closed blindly and transferred in a subcutaneous tunnel up to the mammillary line. Two months later, a presternal skin tube was constructed from local musculocutaneous flaps. Details were published previously (4). After eight years, the cutaneo-colostomy became too narrow, and balloon dilation was unsuccessful. Therefore, a gastrostomy was created, thus feeding became satisfactory. Then a non-healing fistula developed on the skin tube, and a malignant tumor was suspected to be in the background, also responsible for the stenosis, so the skin tube had to be removed (Figure 2A). For reconstruction a 25 cm long free jejunum flap was used supplied by one artery and vein. The conduit was placed presternally so an esophago-jejunal and jejuno-colic anastomosis could be performed both in an end-to-end fashion with one layer 3-0 running absorbable suture. Then a small part of cartilage of the left 4th rib was removed to isolate the internal mammary artery and vein. The arterial anastomosis was made with 8-0 non-absorbable sutures. The venous drainage was established like in the first case, harvesting a saphenous vein graft, connected to the external jugular vein (Figure 2B). The postoperative period was uneventful with good oral feeding. The contrast swallow confirmed a free passage (Figure 2C). The patient lived for decades afterwards and died from another reason at the age of 79.
Median sternotomy route—patient 3
A 45-year-old male committed suicide with hydrochloric acid. In another hospital, an esophago-gasterectomy was performed due to extensive stomach necrosis. The patient survived, and half a year later reconstruction was made with substernally placed right colon. He was discharged from the hospital with good swallowing function, but developed dysphagia after a few weeks, due to an ischemic colonic stricture affecting the entire conduit (Figure 3A). The patient was treated conservatively with dilation, but the effect was unsatisfactory. The lumen could only be kept open with increasing dilating pressure with a high risk of perforation. Therefore, the strictured part of colon was removed through a median sternotomy. A 20 cm jejunum flap supported by one artery and vein was harvested through an abdominal incision. The internal mammary artery was prepared for the vascular anastomosis. The venous outflow was established using a saphenous graft (Figure 3B), which was connected in an end-to-side fashion to the innominate vein. At the contrast swallow intact anastomoses and proper jejunal lumen could be observed. (Figure 3C). The patient recovered without any complications and has been living for eight years with good swallowing function.

Discussion
Colon replacement in esophageal surgery is a routine procedure. When planning an esophageal reconstruction using a colon conduit, it is always advisable to clarify the blood supply of the colon and possible anatomical variations by angiography or angio-CT, because adequate vascularization is an essential requirement to the technical success of the surgery. Anyway, the final decision about the most suitable segment to be harvested is always made at the exploration (5). In most cases, transillumination can be used to assess the anatomical situation. In uncertain cases, the adequacy of the circulation can be checked by temporarily clamping the target vessels and by checking the pulse. The color change of the colon with insufficient blood supply is not reliable since it can only be noted after a longer period unlike small intestine. The signs of venous obstruction are the congestion of the bowel, and gorging of the mesenteric veins. Venous drainage insufficiency may be caused by compression or kinking of the bowel or the vascular pedicle. Postoperative hypotension must be avoided since it can cause complication even after a well-executed operation. Adequate fluid resuscitation is advised and vasopressors should be avoided as much as possible, as vasoconstriction can jeopardize the circulation of the graft.
Conduit necrosis presents with septic symptoms: tachycardia, hypotension, tachypnea, and fever. Contrast CT examination shows no perfusion, and gas bubbles and fluid collection may be presented in its vicinity. The best way to verify necrosis is to open the cervical wound. Endoscopic examination is usually unnecessary. Detection or even suspicion of ischemia is an indication for urgent reoperation (6). If necrosis is confirmed, take-down of the cervical anastomosis and placement of an esophagostomy is required. The substitute should then be retracted from an abdominal approach to resect the necrotic part. The remaining healthy conduit should be closed blindly and placed subcutaneously on the anterior thorax as high as possible. Any length of healthy conduit should be preserved since it simplifies the acute intervention by eliminating the need to close the cologastrostomy. Moreover, the reconstruction will be simpler and would need a shorter segment of free jejunum to overlap the gap. The creation of a colostomy at the level of sternum is not advised, because it requires special care. After removing the necrotic portion, the area must be thoroughly flushed and drained. The loss of transplant is connected to a 30–50% mortality rate (1,6). Reconstruction may take place within 6 to 8 weeks after the recovery while the organ functions and nutritional parameters must return to an acceptable range. This condition can be achieved by carefully planned enteral feeding, possibly combined with partial parenteral nutrition. In the first patient we had to wait for nearly one year since nutritional parameters improved at a slow rate using the jejunostomy only. In the second patient, the conduit necrosis was only about 10 cm long so the remaining part reached up to the mammillary line subcutaneously. Reconstruction with a musculocutaneous flap was performed two months later. At that time, the policy was to have the skin tube as the last resort after two unsuccessful esophageal reconstruction attempts. However, eight years later, due to the development of our surgical techniques, we could solve the stricture of the skin-tube using free jejunal flap transplantation. In the third case, the ischemic colon stricture was detected a few weeks after the operation with unsuccessful dilation attempts. In the latter cases, resection of the defective segments and restoration of continuity with the free jejunum transfer took place in one sitting.
Microvascular augmentation of jejunum flaps or so called “supercharging” method was first proposed in 1947 by Longmire (7) for an ante-thoracic esophageal substitution. After a long gap in 1956, Androsov (8) published this vascular enhancement technique during esophageal reconstruction in 11 patients. Although these early publications demonstrated that long segment esophageal replacement with supercharged jejunum was possible, the technical difficulties of the operation precluded its routine acceptance. Later this method became a routine operation in expert centers. In the 1980s, several successful series of such procedures were published (8-11). The free jejunum transplantation was later mainly used for the reconstruction after pharyngo-laryngectomy (9,10). Our first successful free jejunum transplantation was performed in 1989 and our first series were reported in 2006 (12). In a larger series, free jejunum flap transplantation to replace the thoracic esophagus was reported in 1984 by Kato et al. (13). Further series reported also about the application of supercharged jejunal flaps. Graft failure rate was between 0–9.3%.(14,15). Germain et al. and Takushima et al. reported the first “double arterial pedicled” free jejunum transfer (16,17). Okumura reported on a two-stage operation of an esophageal malignancy. Delayed esophageal reconstruction with free jejunal flap was performed subcutaneously on eleven patients four weeks after subtotal esophagectomy (18). All of the authors used the internal mammary artery and vein for the supercharged or free jejunum flap, to replace the thoracic segment. The donor vessel was accessed by resecting the sternal end of the 3rd rib. We have chosen the 4th rib for isolating the vessels. The height of donor vessel preparation mainly depends on the level of the flap’s pedicle location. Transplantation is always done in an isoperistaltic fashion. In our third case, there was no need for rib resection due to the sternotomy, so the internal mammary artery was reached intrathoracally like in cardiac surgery. Our approaches varied in each case, using substernal, presternal and sternotomy routes.
In the first patient we used a special jejunal flap so called “hybrid supercharged flap”. Despite the ligation of three straight arteries, the flap was not long enough to reach up to the neck. To obtain the required length of the graft, the vascular arch was interrupted between the second and third branch. Thus, the third branch with interrupted circulation was connected to the internal mammary artery as in a free jejunal graft, but the bowel continuity remained untouched with the Roux loop. The jejunum was substernally pulled up to the neck and the interrupted vascular pedicle (3rd branch) could be pulled out through the parasternal window and the microvascular anastomosis was carried out from outside the chest. In all of our 3 cases the internal mammary veins were judged to be too thin; therefore, the venous drainage was established through a saphenous graft used as an extension to interconnect to external jugular or innominate veins. In the literature few hundred successful esophageal replacements are reported with supercharged jejunum flaps (14,15), but substantially less are found about free jejunal transplantations (18,19). Up until now, Iwata et al. and Swisher et al. have reported on jejunal replacements with an interrupted arcade (19,20). Safe execution of the microvascular anastomosis requires fluency and high-volume practice. In expert centers, the success rate is around 90–95% (14,15). There were no complications in our three cases, due to the outstanding experience of the plastic surgical team led by GP gained along more than 300 free flap breast reconstructions.
Overall, it can be stated that following an esophagectomy free jejunal graft transplantation can be used to resolve complicated cases that cannot be reconstructed with any other methods and jejunum offers a good swallowing function and quality of life.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoe.2019.09.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wormuth JK, Heitmiller RF. Esophageal conduit necrosis. Thorac Surg Clin 2006;16:11-22. [Crossref] [PubMed]
- Reslinger V, Tranchart H, D'Annunzio E, et al. Esophageal reconstruction by colon interposition after esophagectomy for cancer analysis of current indications, operative outcomes, and long-term survival. J Surg Oncol 2016;113:159-64. [Crossref] [PubMed]
- Yasuda T, Shiozaki H. Esophageal reconstruction with colon tissue. Surg Today. 2011;41:745-53. [Crossref] [PubMed]
- Horváth OP, Cseke L, Borbély L, et al. Skin tube esophagus: present indications and late malignization. Dis Esophagus 2000;13:251-4. [Crossref] [PubMed]
- Peters JH, Kronson JW, Katz M, et al. Arterial anatomic considerations in colon interposition for esophageal replacement. Arch Surg 1995;130:858-62; discussion 862-3. [Crossref] [PubMed]
- Dickinson KJ, Blackmon SH. Management of Conduit Necrosis Following Esophagectomy. Thorac Surg Clin 2015;25:461-70. [Crossref] [PubMed]
- Longmire WP Jr. A modification of the Roux technique for antethoracic esophageal reconstruction. Surgery 1947;22:94-100. [PubMed]
- Androsov PI. Blood supply of mobilized intestine used for an artificial esophagus. AMA Arch Surg 1956;73:917-26. [Crossref] [PubMed]
- Meyers WC, Seigler HF, Hanks JB, et al. Postoperative function of "free" jejunal transplants for replacement of the cervical esophagus. Ann Surg 1980;192:439-50. [Crossref] [PubMed]
- Hester TR, McConnel FM, Nahal F, et al. Reconstruction of cervical esophagus, hypopharynx and oral cavity using free jejunal transfer. Am J Surg 1980;140:487-91. [Crossref] [PubMed]
- Tizian C, Berger A, Schulz-Coulon HJ, et al. Langenbecks Arch Chir 1985;366:139-43. [Reconstruction of the esophagus and hypopharynx by free jejunum interposition]. [Crossref] [PubMed]
- Pavlovics G, Cseke L, Papp A, et al. Esophagus reconstruction with free jejunal transfer. Microsurgery 2006;26:73-7. [Crossref] [PubMed]
- Kato H, Iizuka T, Watanabe H, et al. Reconstruction of the esophagus by microvascular surgery. Jpn J Clin Oncol 1984;14:379-84. [PubMed]
- Watanabe M, Mine S, Nishida K, et al. Reconstruction after esophagectomy for esophageal cancer patients with a history of gastrectomy. Gen Thorac Cardiovasc Surg 2016;64:457-63. [Crossref] [PubMed]
- Poh M, Selber JC, Skoracki R, et al. Technical challenges of total esophageal reconstruction using a supercharged jejunal flap. Ann Surg 2011;253:1122-9. [Crossref] [PubMed]
- Germain MA, Hartl DM, Boutin P, et al. Total esophagoplasty using a doubly vascularized free jejunal transplant: a last resort in two patients. Plast Reconstr Surg 2003;111:801-4. [Crossref] [PubMed]
- Takushima A, Momosawa A, Asato H, et al. Double vascular pedicled free jejunum transfer for total esophageal reconstruction. J Reconstr Microsurg 2005;21:5-10. [Crossref] [PubMed]
- 18 Okumura Y, Mori K, Yamagata Y, et al. Two-stage operation for thoracic esophageal cancer: esophagectomy and subsequent reconstruction by a free jejunal flap. Surg Today 2014;44:395-8.
- Iwata N, Koike M, Kamei Y, et al. Antethoracic pedicled jejunum reconstruction with the supercharge technique for esophageal cancer. World J Surg 2012;36:2622-9. [Crossref] [PubMed]
- Swisher SG, Hofstetter WL, Miller MJ. The supercharged microvascular jejunal interposition. Semin Thorac Cardiovasc Surg 2007;19:56-65. [Crossref] [PubMed]
Cite this article as: Horvath OP, Abedini N, Papp A, Vereczkei A, Pavlovics G. Free jejunal flap esophagoplasty for ischemic colon conduit replacement. Ann Esophagus 2019;2:15.


