Oesophagectomy with en bloc resection of the thoracic duct: risk factors for post-operative chyle leak and current management
Introduction
Chylothorax following oesophagectomy for cancer is reported to occur in 0–11% of patients (1,2). The development of a chyle leak (CL) has a detrimental impact on survival, disease recurrence, length of hospital stays and postoperative quality of life (3-9). There are 2 arguments for routine ligation and resection of the thoracic duct. Firstly, on an oncological basis, en bloc resection of the thoracic duct during oesophagectomy for oesophageal cancer has been reported to increase the number of resected mediastinal lymph nodes and ensures removal of potentially involved nodes adjacent to the duct (10). Secondly, to prevent development of a chylothorax, the prophylactic mass ligation of the thoracic duct has been recommended with a randomized trial of 653 patients undergoing oesophageal resection reporting a chylothorax rate of 0.3% with ligation of the duct and 2.1% in patients with duct preservation (11). However, 2 recent systematic reviews and meta-analysis have reached different conclusions (12,13). Crucitti et al. reported a significant reduction in chylothorax rates with prophylactic ligation of the thoracic duct while Lei et al. found no evidence to support such an approach. Current practice in our unit is to routinely ligate the thoracic duct and remove all surrounding tissue en bloc.
The aim of this study was to determine the rate of chylothorax within the unit and to identify patient, disease and surgical risk factors which predispose to CL following oesophagectomy.
Methods
All patients who underwent subtotal oesophagectomy with formation of a gastric conduit and oesophago-gastric anastomosis were included in a 3-year study period (January 2012–December 2014). Patients were identified from a prospectively collected database in a single tertiary referral centre. All operations were performed or supervised by 5 consultant specialist oesophago-gastric surgeons. Patients who underwent transhiatal and left thoraco-abdominal procedures were excluded. Patients who developed anastomotic leak were excluded. Case notes were individually retrieved and reviewed retrospectively. All patients had placement of a feeding jejunostomy and feeding was commenced at 48 hours postoperatively at 10 mL per hour then increased incrementally over the next few days using a standard protocol.
CL was diagnosed clinically in the majority of cases by the appearance of creamy hydrophobic fluid in the chest drain in the post-operative period on commencing enteral feeding. If confirmation was required drain fluid was tested for the presence of chylomicrons or for a triglyceride level greater than 100 mg/dL.
Statistical analysis was undertaken using SPSS version 21 and STATA version 13.1. Fisher’s exact test was used to determine the association between outcome and categorical variables. Continuous variables were assessed for t-test assumptions, equal variance between groups, normal distribution, no significant outliers, by graphing and calculating descriptive statistics. If violations were identified data were log transformed to fit a normal distribution and/or an unequal variance design used. If non-normal distribution Mann-Whitney U test was used as a non-parametric test. P values of less than 0.05 was considered statistically significant.
Ethical approval was granted by the local Caldicott Guardian Council prior to data collection. All participants gave informed consent for anonymised data to be used for clinical research purposes at the time of surgery.
Results
During the 36-month study period 147 oesophagectomies were performed. Eighteen patients with anastomotic leak were excluded. Of the remaining 129 patients, 11 developed a CL (8.5%). A total of 10 patients underwent thoracoscopic-assisted oesophagectomy with a cervical anastomosis and 119 an Ivor-Lewis 2-phase oesophagectomy with anastomosis in the upper thorax. The patient demographics are described in Table 1.
Table 1
| Demographics | Control (N=118) | Chyle leak (N=11) | P value |
|---|---|---|---|
| Median age in yrs [IQR] | 65 [58–72] | 67 [61–77] | 0.24 |
| M:F | 96:22 | 7:4 | 0.76 |
| ASA [IQR] | 2 [2–3] | 2 [2–2] | |
| BMI, mean (range) | 27.5 (24.0–30.3) | 24.1 (23.0–28.5) | 0.098 |
| CL (%) | |||
| CL in smokers | 4/25 (16.0) | 0.22 | |
| CL in non-smokers | 7/104 (6.7) | ||
| CL with neoadj chemo | 7/62 (11.3) | 0.35 | |
| CL without neoadj chemo | 4/67 (6.0) | ||
Neoadj chemo, neoadjuvant chemotherapy; ASA, American Society of Anesthesiologists; CL, chyle leak.
Patients with squamous cell carcinoma had a significantly increased risk of CL (6/16, 37.5%) when compared to patients with other tumour types (5/113, 4.4%, P=0.0005, Table 2). Patients with low BMI were more likely to develop a CL although this was not statistically significant (Table 1). Neither the extent of lymphadenectomy nor the extent of lymph node involvement (N stage) were associated with CL (Table 2).
Table 2
| Demographics | Control (N=118) (%) | Chyle leak (N=11) (%) | P value |
|---|---|---|---|
| Tumour location | – | ||
| Middle third | 10/118 (8.5) | 1/11 (9.1) | |
| Lower third | 81/118 (68.6) | 10/11(90.9) | |
| OGJ 1 | 13/118 (11.0) | 0/11 (0) | |
| OGJ 2 | 14/118 (11.9) | 0/11 (0) | |
| Pathology | |||
| Adenocarcinoma | 96/118 (81.4) | 5/11 (45.5) | |
| Squamous | 10/118 (8.5) | 6/11 (54.5) | 0.0005 |
| Other | 12/118 (10.2) | 0/11 (0) | |
| Staging | – | ||
| Tx | 1/118 (0.8) | 0/11 (0) | |
| T1 | 36/118 (30.5) | 0/11 (0) | |
| T2 | 14/118 (11.9) | 1/11 (9.1) | |
| T3 | 61/118 (51.7) | 9/11 (81.8) | |
| T4 | 5/118 (4.2) | 1/11 (9.1) | |
| Melanoma | 1/118 (0.8) | 0/11 (0) | |
| Nodal stage | – | ||
| N0 | 51/118 (43.2) | 4/18 (22.2) | |
| N1 | 31/118 (26.3) | 5/18 (27.8) | |
| N2 | 23/118 (19.5) | 5/18 (27.8) | |
| N3 | 12/118 (10.2) | 4/18 (22.2) | |
| Melanoma | 1/118 (0.8) | 0/18 (0) | |
| Neurovascular invasion (yes) | 74/118 (62.7) | 9/11 (81.8) | – |
| Mean No. of LNs examined, [range] | 20.9 [5–50] | 25 [9–40] | 0.17 |
| Mean No. of LNs positive, [range] | 2.3 [0–18] | 4.9 [0–23] | 0.18 |
OGJ, oesophagogastric junction.
Patients who developed a CL had both a prolonged ICU stay compared to those without a CL [8.3 days (5–16 days) vs. 5.8 days (2–19 days), P=0.01] and a prolonged total inpatient stay [24.5 days (14–62 days) vs. 18.2 days (9–84 days), P=0.01] (Table 3).
Table 3
| Demographics | Control (N=118) (%) | Chyle leak (N=11) (%) | P value |
|---|---|---|---|
| Mean total intraoperative blood loss [range], mL | 560.2 [50–3,900] | 399.1 [200–700] | 0.30 |
| Mean total operating time [range], min | 391.2 [380–540] | 367.7 [300–500] | 0.91 |
| Stapled anastomosis (yes) | 74/118 (62.7) | 8/11 (72.7) | 0.76 |
| Lap gastric mobilisation (yes) | 14/118 (11.9) | 2/11 (18.2) | – |
| Lap oesophageal mobilisation (yes) | 10/118 (8.5) | 0/11 (0) | – |
| Abdo phase performed by trainee (yes) | 64/118 (54.2) | 4/11 (36.4) | – |
| Chest phase performed by trainee (yes) | 53/118 (44.9) | 6/11 (54.5) | – |
| Mean ICU stay ventilated [range], days | 1.8 [0–55] | 0 [0–0] | 0.23 |
| Mean ICU stay unventilated [range], days | 5.8 [2–19] | 8.3 [5–16] | 0.01 |
| Mean duration of admission [range], days | 18.2 [9–84] | 24.5 [14–62] | 0.01 |
| New AF (yes) | 15/118 (12.7) | – | – |
AF, atrial fibrillation.
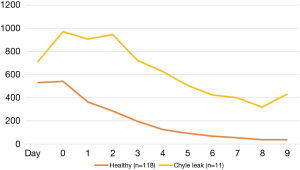
On day 1 post-operatively both groups of patients had similar median chest drain volumes, median volume 715.5 mL (200–1,550 mL) in patients who subsequently developed a CL and 532.1 mL (25–1,025 mL) in patients who did not (P=0.08) (Table 4). In comparison on day 2, prior to commencing enteral feeding, patients who subsequently developed a CL had a significantly higher chest drain volume compared to those who did not, 970.5 mL (100–2,000 mL) vs. 541.2 mL (125–4,225 mL), P=0.003. Using a chest drain volume threshold of 600 mL on day 2, prior to commencing enteral feeding or the appearance of chyle, the sensitivity and specificity for predicting a CL was 92% and 82% respectively.
Table 4
| Day | Control (N=118) | Chyle leak (N=11) | P value |
|---|---|---|---|
| Day 0 | 532.1 [25–1,025] | 715.5 [200–1,550] | 0.08 |
| Day 1 | 541.2 [125–4,225] | 970.5 [100–2,000] | 0.003 |
| Day 2 | 364.0 [25–1,225] | 907.3 [300–1,800] | <0.0001 |
| Day 3 | 286.7 [25–825] | 944.6 [150–2,275] | 0.0001 |
| Day 4 | 195.9 [0–700] | 724.1 [235–1,575] | <0.0001 |
| Day 5 | 126.1 [0–565] | 627.3 [175–1,500] | <0.0001 |
| Day 6 | 93.1 [0–400] | 509.1 [25–1,625] | <0.0001 |
| Day 7 | 69.1 [0–350] | 425.0 [0–1,900] | 0.0003 |
| Day 8 | 53.7 [0–350] | 400.0 [30–1,020] | 0.0001 |
| Day 9 | 38.2 [0–225] | 320.0 [0–725] | <0.0001 |
| Day 10 | 37.3 [0–200] | 430.6 [0–925] | <0.0001 |
Six of the 11 (54.5%) patients with a CL had a reduction in chest drainage to <600 mL/24 h after introduction of conservative measures (Table 5). All were successfully managed with non-operative treatment. Five patients with high volume CL (>600 mL/24 h despite conservative management) underwent re-thoracotomy. At the time of re-operation, the main thoracic duct was never the source of leakage with numerous lymphatic channels throughout the mediastinal region of dissection the source. These were controlled with insertion of sutures at multiple levels. This achieved control in 4 cases with 1 patient subsequently requiring the insertion of a pleura-peritoneal shunt.
Table 5
| Patients | Chyle volume | Modality of treatment | Intraoperative findings | Resolution |
|---|---|---|---|---|
| 1 | Low | Conservative | N/A | Yes |
| 2 | Low | Conservative | N/A | Yes |
| 3 | Low | Conservative | N/A | Yes |
| 4 | Low | Conservative | N/A | Yes |
| 5 | Low | Conservative | N/A | Yes |
| 6 | Low | Conservative | N/A | Yes |
| 7 | High | Re-thoracotomy | Leaks at multiple levels | Yes |
| 8 | High | Re-thoracotomy | Leaks at multiple levels | Yes |
| 9 | High | Re-thoracotomy | Leaks at multiple levels | Yes |
| 10 | High | Re-thoracotomy | Leaks at multiple levels | Yes |
| 11 | High | Re-thoracotomy | Leaks at multiple levels. Pleuroperitoneal shunt | Yes |
Discussion
The routine practice of ligation of the thoracic duct has not prevented the development of chylothorax post-operatively in our unit. While the leak rate is 8.5% overall, the risk of developing a leak is greatly increased in patients with squamous cell carcinoma. The reason for this is unclear but may relate to lymphatic changes occurring in squamous cell carcinoma that increase the likelihood of a CL. The knowledge that at the time of re-operation the source of the leak was never the main thoracic duct encourages a conservative approach to CL in low volume cases, taken as <600 mL/24 h in our unit. This includes initially changing from a standard enteral feed to a medium-chain triglyceride (MCT) feed and then to total parenteral nutrition (TPN) if chest drain volumes do not decrease quickly. Based on these criteria all patients selected for conservative management were successfully treated. A chest drainage of >600 mL/24 h despite appropriate conservative measures is best treated with a re-thoracotomy at an early stage. The mediastinal lymphatic channels causing the CL are often sizeable and can be successfully controlled with additional sutures in the majority of cases. Fifty mL of cream is administered via the jejunostomy just prior to surgery to aid the identification of the lymphatic channels.
In post oesophagectomy patients the daily chest drainage volume is reported to be highest on the first post-operative day and then reduces daily over time (14). This is similar in our study with patients who did not develop either a chyle or anastomotic leak (N=118) (Figure 1). In contrast, patients who developed a CL had higher chest drain volumes on day 1 and the volumes remained high on the second post-operative day prior to starting enteral feeding or the appearance of chyle. This should raise a high level of suspicion particularly in those with squamous cell cancer of a possible impending problem.
Previous studies have shown that patients with low BMI, squamous cell carcinoma and advanced nodal disease (high number of positive lymph nodes) predispose to a CL (15-17). The results of this study would suggest that neither the radicality of lymph node dissection nor extent of lymph node involvement increased the risk of CL.
In conclusion, this study demonstrates squamous cell carcinoma remains a significant risk factor for the development of post-oesophagectomy chylothorax despite the routine ligation of the thoracic duct. In the knowledge that with this approach the main thoracic duct is never the source of CL, patients with a chest drainage of <600 mL/24 h can be successfully treated conservatively. Patients with a chest drain volume of >600 mL/24 h despite conservative management should under re-thoracotomy at an early stage.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoe.2019.08.04). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethical approval was granted by the local Caldicott Guardian Council prior to data collection. All participants gave informed consent for anonymised data to be used for clinical research purposes at the time of surgery.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Blencowe NS, Strong S, McNair AG, et al. Reporting of short-term clinical outcomes after esophagectomy: a systematic review. Ann Surg 2012;255:658-66. [Crossref] [PubMed]
- Cagol M, Ruol A, Castoro C, et al. Prophylactic thoracic duct mass ligation prevents chylothorax after transthoracic esophagectomy for cancer. World J Surg 2009;33:1684-6. [Crossref] [PubMed]
- Rizk NP, Bach PB, Schrag D, et al. The impact of complications on outcomes after resection for esophageal and gastroesophageal junction carcinoma. J Am Coll Surg 2004;198:42-50. [Crossref] [PubMed]
- Lagarde SM, de Boer JD, ten Kate FJ, et al. Postoperative complications after esophagectomy for adenocarcinoma of the esophagus are related to timing of death due to recurrence. Ann Surg 2008;247:71-6. [Crossref] [PubMed]
- Lerut T, Moons J, Coosemans W, et al. Postoperative complications after transthoracic esophagectomy for cancer of the esophagus and gastroesophageal junction are correlated with early cancer recurrence: role of systematic grading of complications using the modified Clavien classification. Ann Surg 2009;250:798-807. [Crossref] [PubMed]
- Hii MW, Smithers BM, Gotley DC, et al. Impact of postoperative morbidity on long-term survival after oesophagectomy. Br J Surg 2013;100:95-104. [Crossref] [PubMed]
- Derogar M, Orsini N, Sadr-Azodi O, et al. Influence of major postoperative complications on health-related quality of life among long-term survivors of esophageal cancer surgery. J Clin Oncol 2012;30:1615-9. [Crossref] [PubMed]
- Scarpa M, Saadeh LM, Fasolo A, et al. Health-related quality of life in patients with oesophageal cancer: analysis at different steps of the treatment pathway. J Gastrointest Surg 2013;17:421-33. [Crossref] [PubMed]
- Strik C, ten Broek RP, van der Kolk M, et al. Health-related quality of life and hospital costs following esophageal resection: a prospective cohort study. World J Surg Oncol 2015;13:266. [Crossref] [PubMed]
- Matsuda S, Takeuchi H, Kawakubo H, et al. Clinical outcome of transthoracic esophagectomy with thoracic duct resection: Number of dissected lymph node and distribution of lymph node metastasis around the thoracic duct. Medicine (Baltimore) 2016;95:e3839 [Crossref] [PubMed]
- Lai FC, Chen L, Tu YR, et al. Prevention of chylothorax complicating extensive esophageal resection by mass ligation of thoracic duct: a random control study. Ann Thorac Surg 2011;91:1770-4. [Crossref] [PubMed]
- Crucitti P, Mangiameli G, Petitti T, et al. Does prophylactic ligation of the thoracic duct reduce chylothorax rates in patients undergoing oesophagectomy? A systematic review and meta-analysis. Eur J Cardiothorac Surg 2016;50:1019-24. [Crossref] [PubMed]
- Lei Y, Feng Y, Zeng B, et al. Effect of Prophylactic Thoracic Duct Ligation in Reducing the Incidence of Postoperative Chylothorax during Esophagectomy: A Systematic Review and Meta-analysis. Thorac Cardiovasc Surg 2018;66:370-5. [Crossref] [PubMed]
- Kosugi S, Kanda T, Yajima K, et al. Risk factors influencing the pleural drainage volume after transthoracic oesophagectomy. Eur J Cardiothorac Surg 2013;43:1116-20. [Crossref] [PubMed]
- Miao L, Zhang Y, Hu H, et al. Incidence and management of chylothorax after esophagectomy. Thorac Cancer 2015;6:354-8. [Crossref] [PubMed]
- Lagarde SM, Omloo JM, de Jong K, et al. Incidence and management of chyle leakage after esophagectomy. Ann Thorac Surg 2005;80:449-54. [Crossref] [PubMed]
- Shah RD, Luketich JD, Schuchert MJ, et al. Postesophagectomy chylothorax: incidence, risk factors, and outcomes. Ann Thorac Surg 2012;93:897-903; discussion 903-4. [Crossref] [PubMed]
Cite this article as: Ip B, Ng KT, Packer S, Paterson-Brown S, Couper G. Oesophagectomy with en bloc resection of the thoracic duct: risk factors for post-operative chyle leak and current management. Ann Esophagus 2019;2:13.